How to find the structure and classification of Ravulizumab?
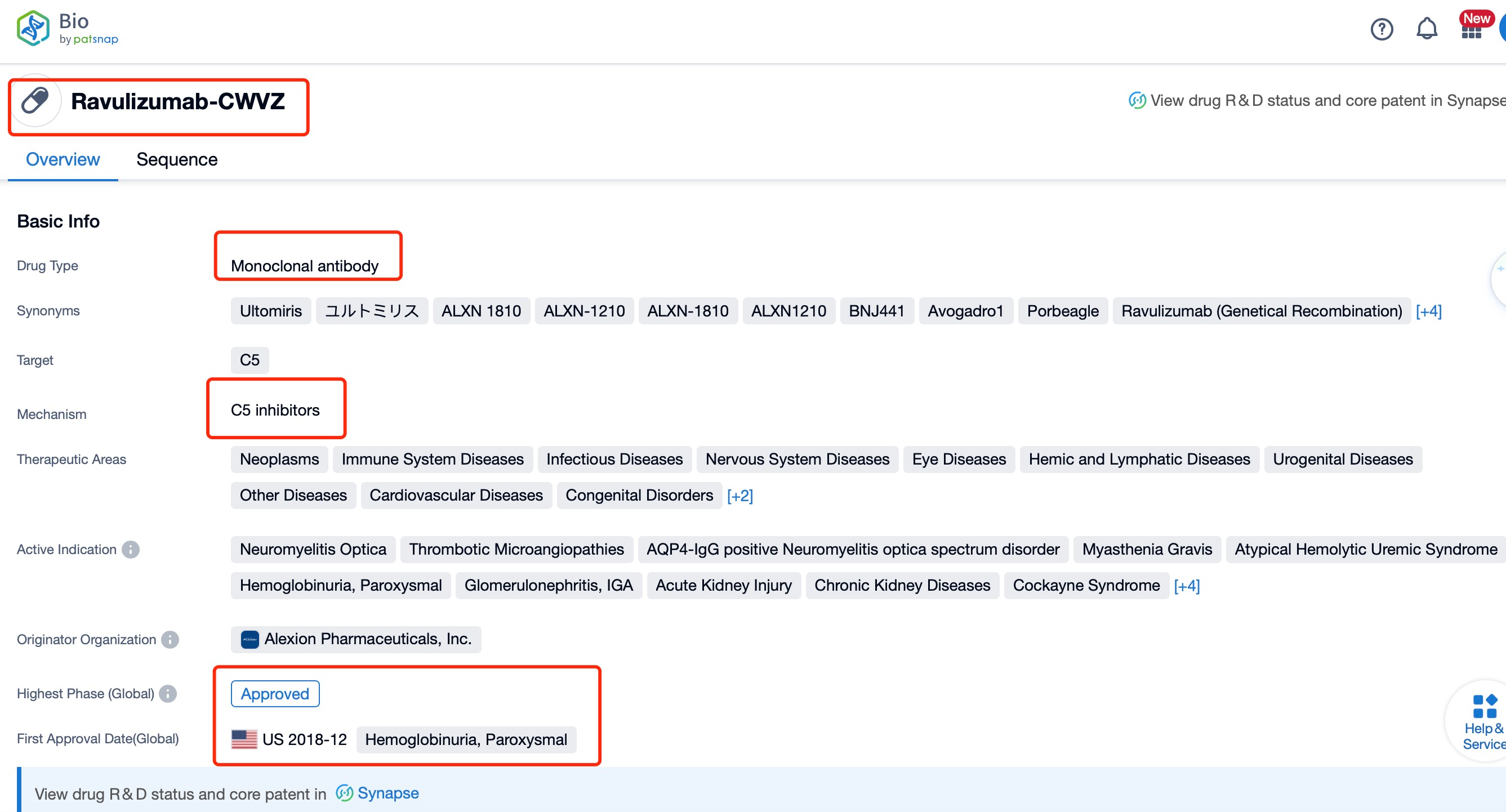
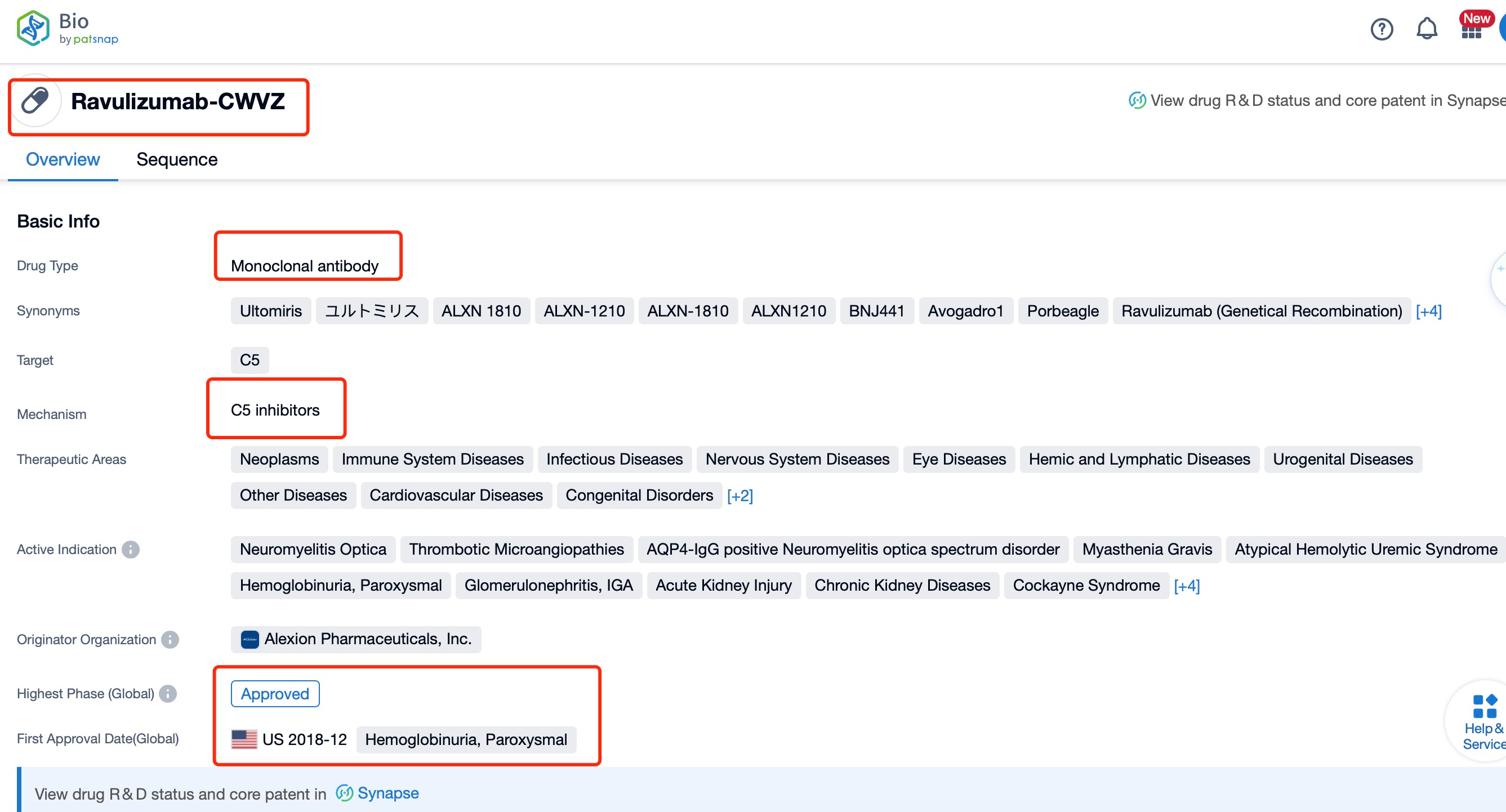
Ravulizumab, a long-acting C5 complement inhibitor, has been developed by Alexion Pharmaceuticals, a leader in complement biology research. This humanized monoclonal antibody targets the C5 protein of the complement system, which plays a crucial role in the immune response. The drug has been indicated for the treatment of various autoimmune diseases, including Paroxysmal Nocturnal Hemoglobinuria (PNH), atypical Hemolytic Uremic Syndrome (aHUS), and Generalized Myasthenia Gravis (gMG). The research institution behind this innovation is Alexion Pharmaceuticals, which has a strong track record in developing complement inhibitors. Ravulizumab is a significant advancement in the treatment of complement-mediated diseases, offering a more convenient dosing schedule compared to previous complement inhibitors.
Summary of Research Progress:
Ravulizumab is a humanized monoclonal antibody that functions as a terminal complement C5 inhibitor. It was developed by Alexion Pharmaceuticals and has been approved for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). The drug works by binding to the complement protein C5, preventing its cleavage into C5a and C5b, which in turn stops the formation of the terminal complement complex C5b-9 that mediates cell lysis.
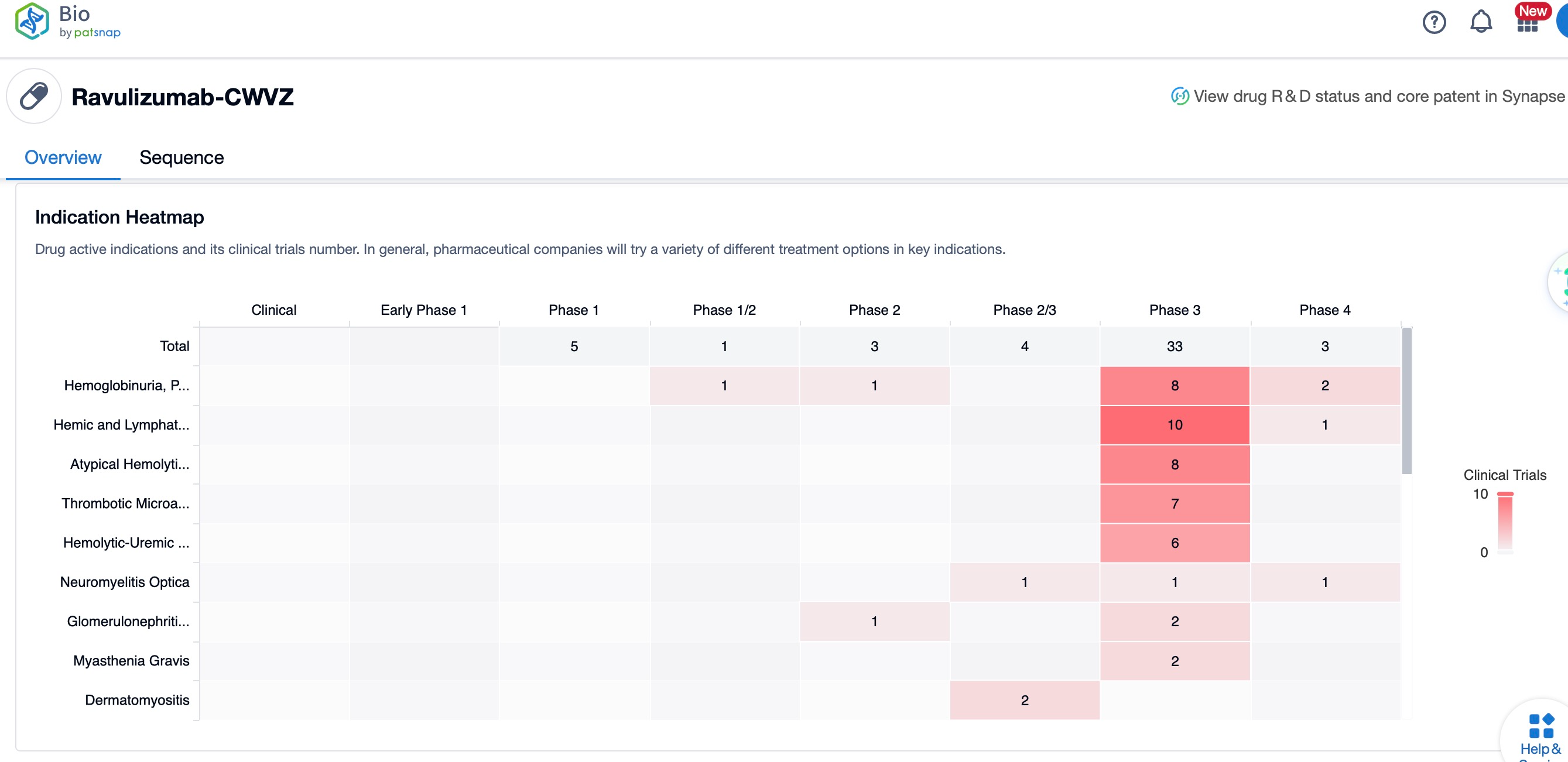
Ravulizumab's mechanism of action involves inhibiting the C5 protein, which is part of the complement system, thus preventing the formation of the terminal complement complex C5b-9 that leads to cell lysis. This drug has been globally listed for the treatment of PNH and aHUS, with additional studies exploring its efficacy in other diseases like IgA nephropathy. The global competitive landscape includes other complement inhibitors such as Eculizumab, which is also a C5 inhibitor but with a shorter duration of action compared to ravulizumab. Clinical research status shows that ravulizumab is in various phases of trials for different indications, with some having reached phase III, demonstrating its potential impact on the treatment landscape of complement-mediated diseases. The drug's extended half-life allows for less frequent dosing, which is a significant advantage over other complement inhibitors, improving patient compliance and quality of life.
Globally, ravulizumab has been marketed and is under regulatory review for additional indications such as myasthenia gravis and IgA nephropathy. The drug has demonstrated efficacy in reducing relapse rates and improving clinical outcomes in patients with NMOSD, with a favorable safety profile that includes a lower risk of infections compared to other complement inhibitors like eculizumab.
In terms of clinical research status, ravulizumab has shown promising results in phase III trials for the treatment of aHUS and is being evaluated in early-phase and preclinical studies for myasthenia gravis and IgA nephropathy, respectively. The drug's development includes a subcutaneous formulation, which is designed to further extend the dosing interval, providing convenience for patients.
The global competitive landscape for ravulizumab includes other complement inhibitors like eculizumab, which is also a C5 inhibitor but with a shorter duration of action. Ravulizumab's extended half-life, due to Xencor's antibody half-life prolongation technology (Xtend™), allows for less frequent dosing, which is a significant advantage over other complement inhibitors and improves patient compliance and quality of life.
Type of Immunoglobulin of Ravulizumab
Ravulizumab belongs to the immunoglobulin G (IgG) class, specifically the IgG4.
Ravulizumab is a humanized monoclonal antibody that belongs to the immunoglobulin G (IgG) class, specifically the IgG4 subtype. This class of antibodies is known for its long serum half-life and ability to activate the complement system, making it an ideal choice for a drug designed to inhibit the complement cascade. The IgG4 subtype is particularly useful in this context because it has a longer half-life compared to other IgG subtypes, which is beneficial for a drug like ravulizumab that is intended to provide sustained inhibition of the complement system.
Another key advantage of IgG4 antibodies is their reduced capacity to activate the complement system and interact with Fc gamma receptors. Unlike other IgG subtypes, IgG4 has a diminished ability to bind to these receptors, which are involved in antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). This reduced interaction helps minimize the risk of unwanted inflammatory responses and potential side effects, contributing to a safer therapeutic profile. For patients receiving ravulizumab, this translates into a treatment that is not only effective but also less likely to cause adverse reactions.
Moreover, the structure of IgG4 antibodies facilitates a unique mechanism known as Fab arm exchange, where the antigen-binding fragments (Fabs) can switch between two monomers within a dimeric structure. This property may contribute to the overall stability of the molecule and could play a role in modulating its interaction with antigens. Combined with its long half-life and reduced effector function, these structural features make IgG4 antibodies highly desirable for treatments that require sustained and controlled immune modulation, such as those for rare hematological disorders like PNH and aHUS.
Light and Heavy Chains and Structural Characteristics of Ravulizumab
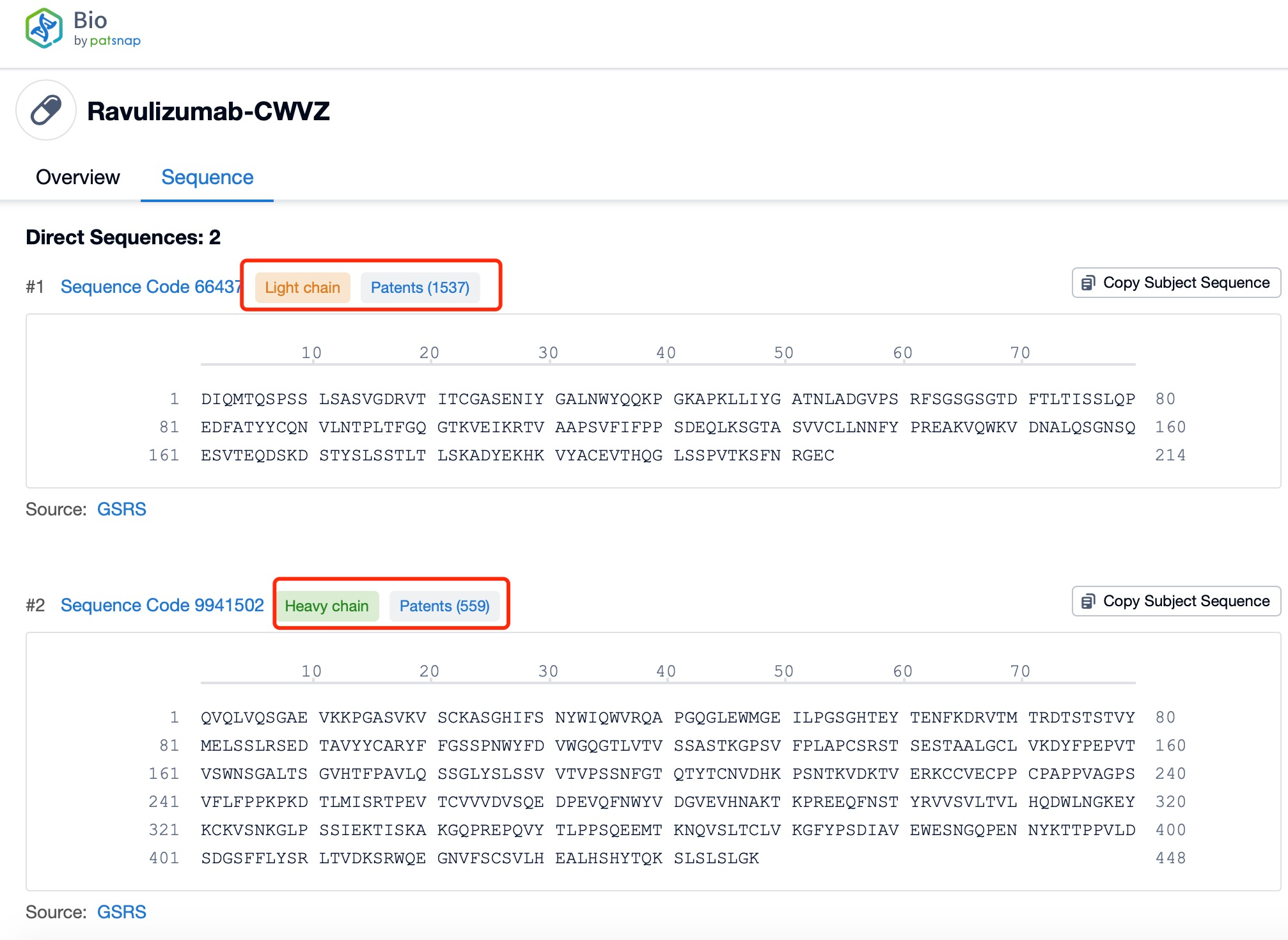
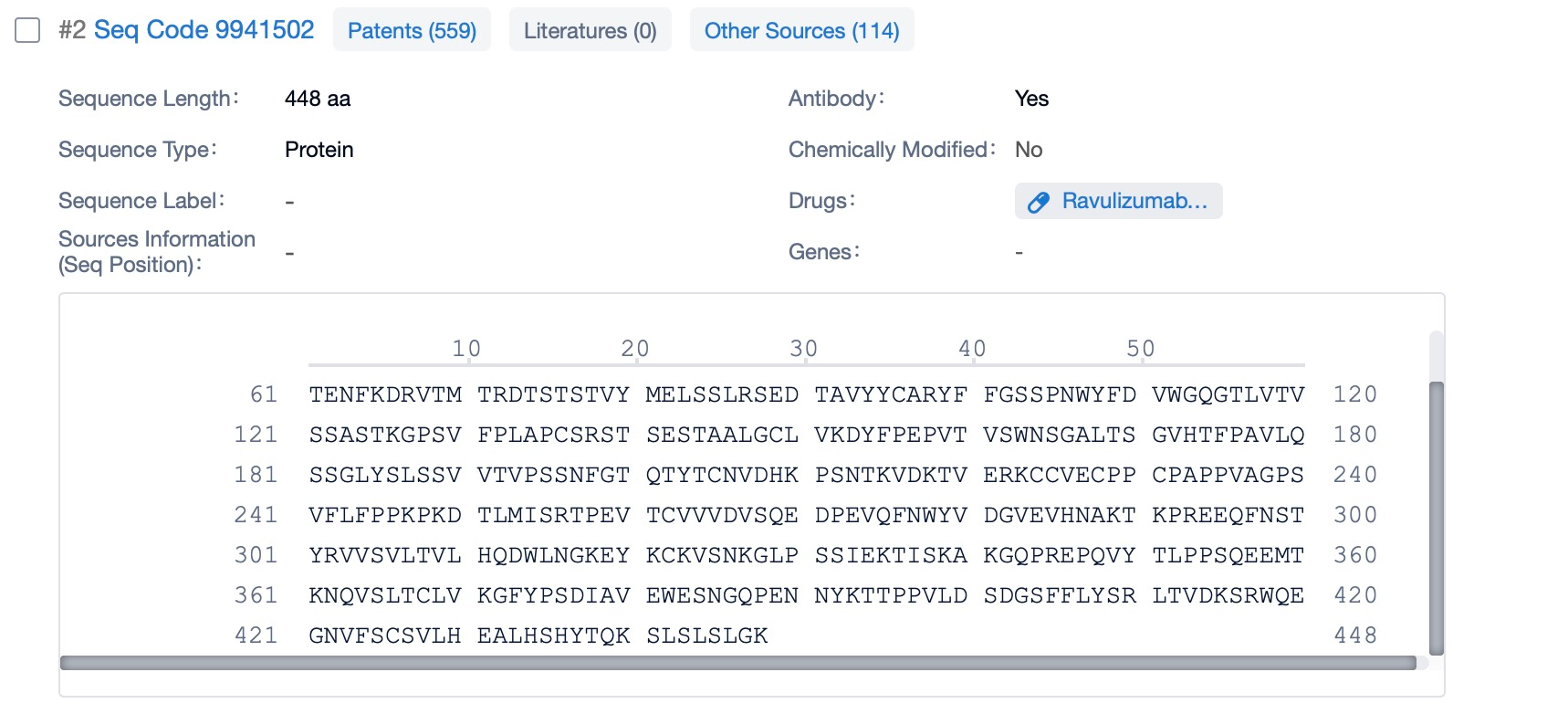
Ravulizumab is composed of two types of polypeptide chains, light and heavy chains, which are typical of monoclonal antibodies. The light chains of ravulizumab are approximately 214 amino acids in length and are encoded by genes located on human chromosomes 2 and 22. The heavy chains, which are larger, consist of about 450 amino acids and are encoded by genes on chromosome 14. The heavy chain includes the Fc region, which is a significant part of the antibody for its effector functions. Ravulizumab's structural characteristics include its humanized nature, which reduces immunogenicity and increases its safety profile. The antibody is designed to have high affinity and specificity for the C5 protein, ensuring that it can effectively inhibit the complement cascade.
Summary and Prospect
Ravulizumab represents a significant advancement in the field of complement inhibition, offering a long-acting alternative to existing therapies. Its unique structure and mechanism of action provide a new option for patients with complement-mediated diseases. The prospect for ravulizumab is promising, with ongoing clinical trials exploring its potential in a range of indications. As research continues, it is expected that ravulizumab will play an increasingly important role in the treatment of various autoimmune and complement-mediated disorders, improving patient outcomes and quality of life.
How to find the structure and classification of antibody drugs?
In Patsnap Bio, you can find the sequence and latest research and development advances of all antibody drugs.
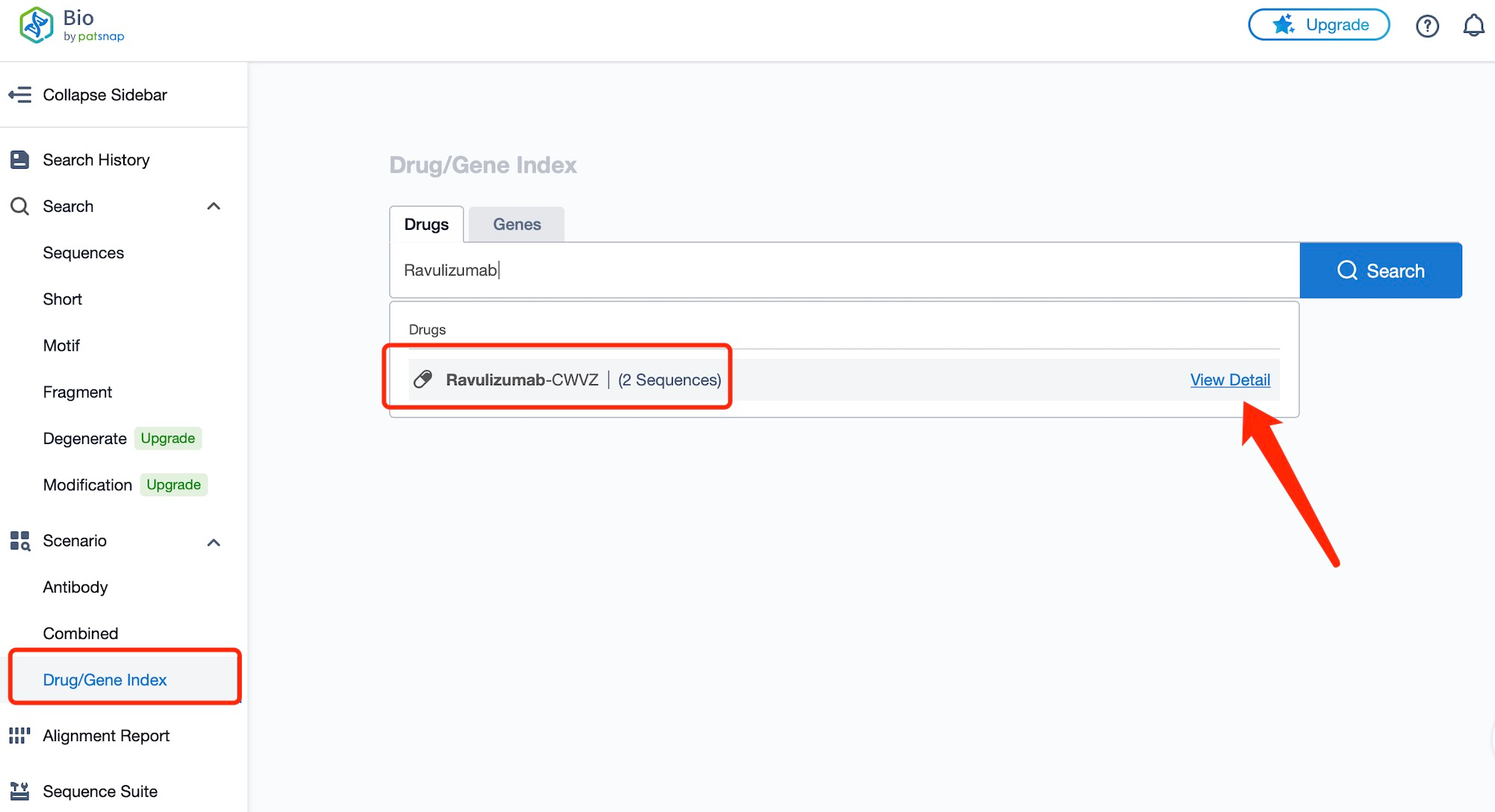
Taking ravulizumab as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' Ravulizumab ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of ravulizumab.
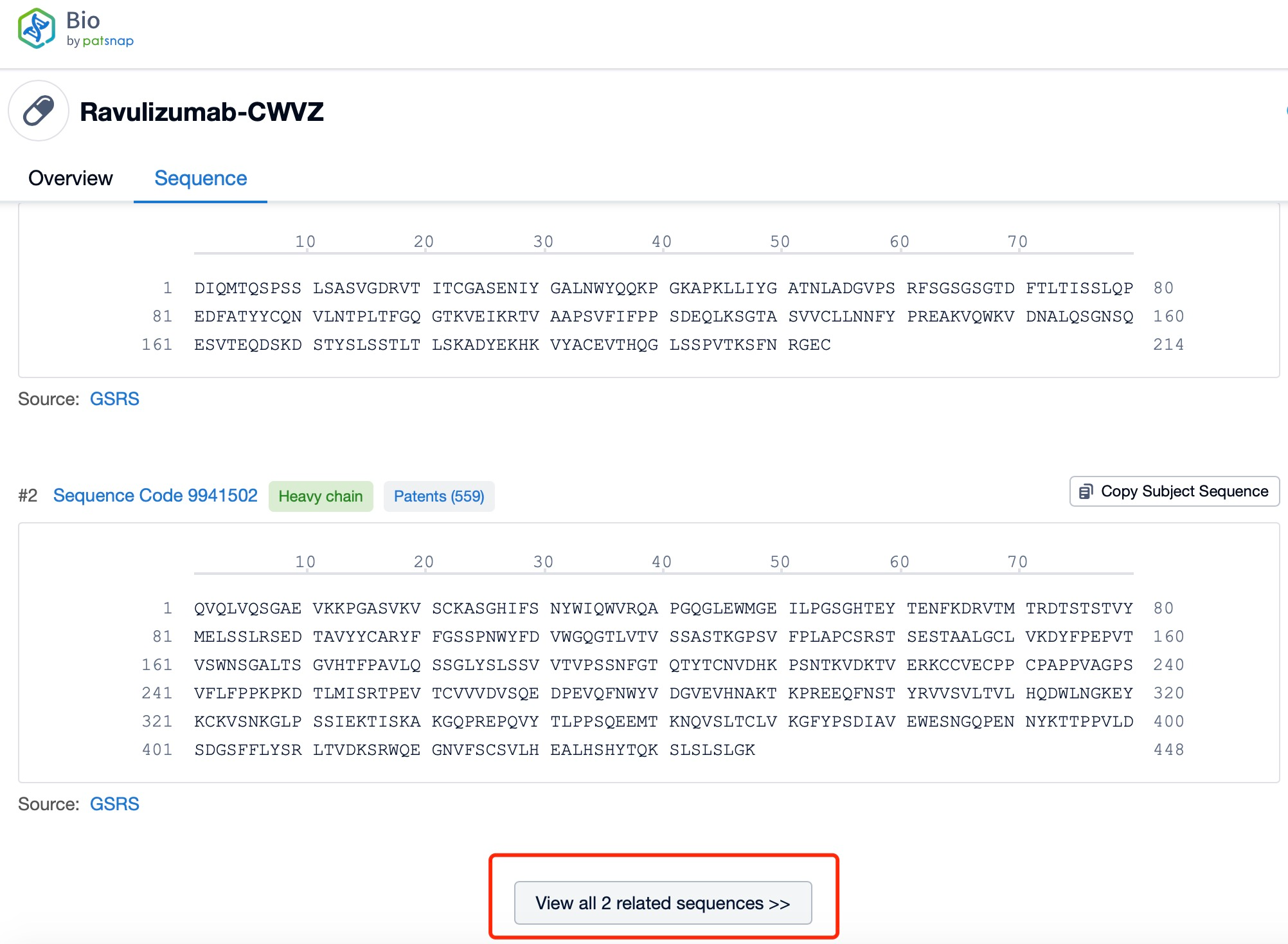
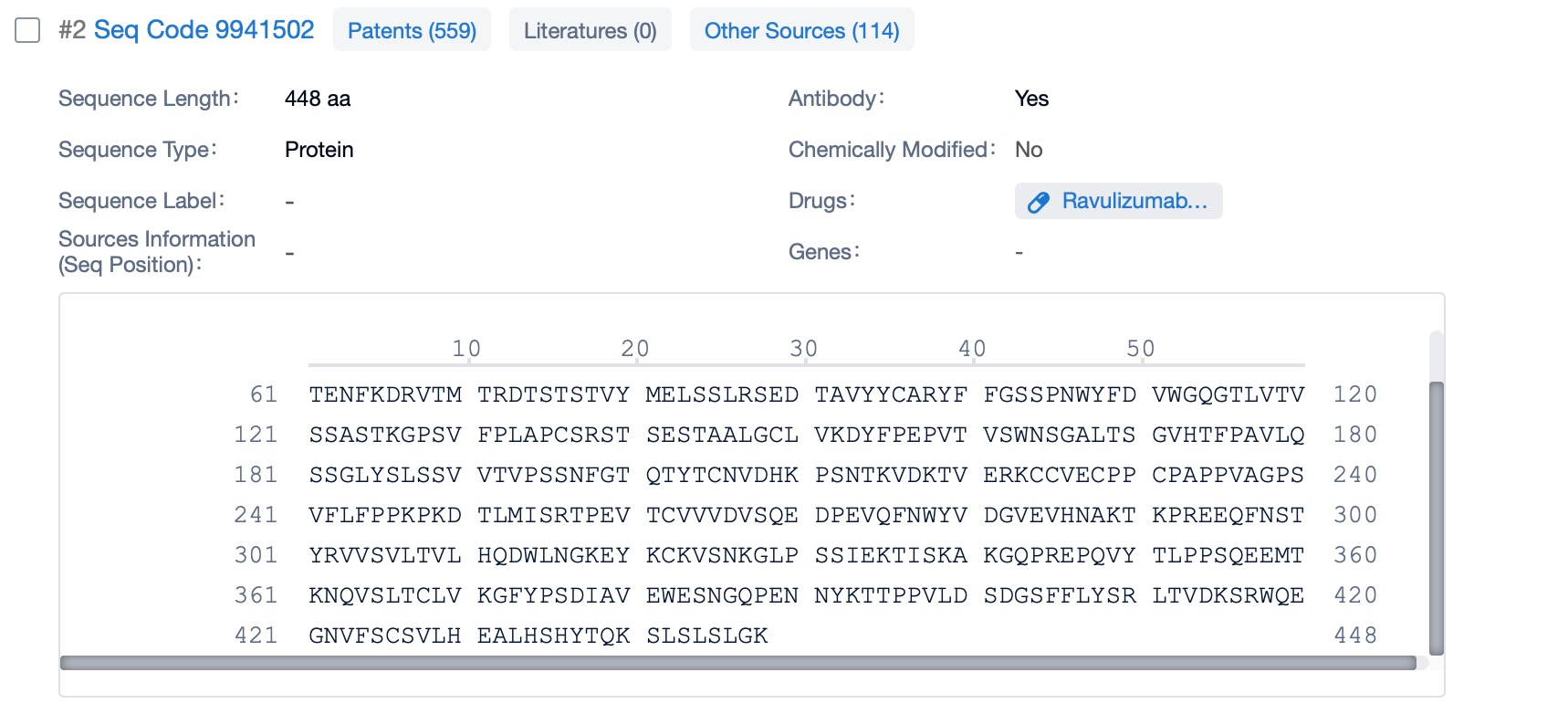
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
Clicking on the sequence name will provide you with all the basic information of that sequence. Here, you can easily query the sequence and action of the light and light chains of antibodies.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.