Huonslab Reports Positive In Vivo PK Results for Trastuzumab/HyDIFFUZETM vs. Standard Trastuzumab and rHuPH20 Co-formulation
Huonslab, Co. Ltd. ("Huonslab"), a division under Huons Global (KOSDAQ: 084110), has revealed encouraging outcomes from an In Vivo comparative pharmacokinetic (PK) study. The study affirmed the biological equivalence between trastuzumab/HYDIFFUZE™ and trastuzumab/Enhanze.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Huonslab previously validated the equivalent non-clinical effectiveness of HYDIFFUZE™ compared to Enhanze through a dye dispersion assay in a mouse model, demonstrating it as a locally acting and transient permeation enhancer. Additionally, biological equivalence between rituximab/HYDIFFUZE™ and rituximab/Enhanze was confirmed in a comparative PK study in rats.
Dr. Young Sun Lee, Chief Business Officer at Huonslab, mentioned, “The substance patent for Enhanze has already expired in Europe and South Korea and will expire in the United States in 2027, based on verification by our external patent law firm.”
“With comprehensive FTO analysis and Huonslab's own process patent, we are equipped with unparalleled excellence and rigorous scientific documentation to establish ourselves as a global leader in high concentration antibody formulation (HCAF) for subcutaneous delivery.”
Lee concluded, “We are speeding up the pivotal Phase 1 trial of Hydizyme™, a stand-alone human hyaluronidase drug product, in South Korea, aiming to secure the Biologics License Application (BLA) with MFDS in 2026”
Huonslab will be presenting at the company presentation session during the upcoming BIO Europe 2024 in Stockholm, Sweden.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
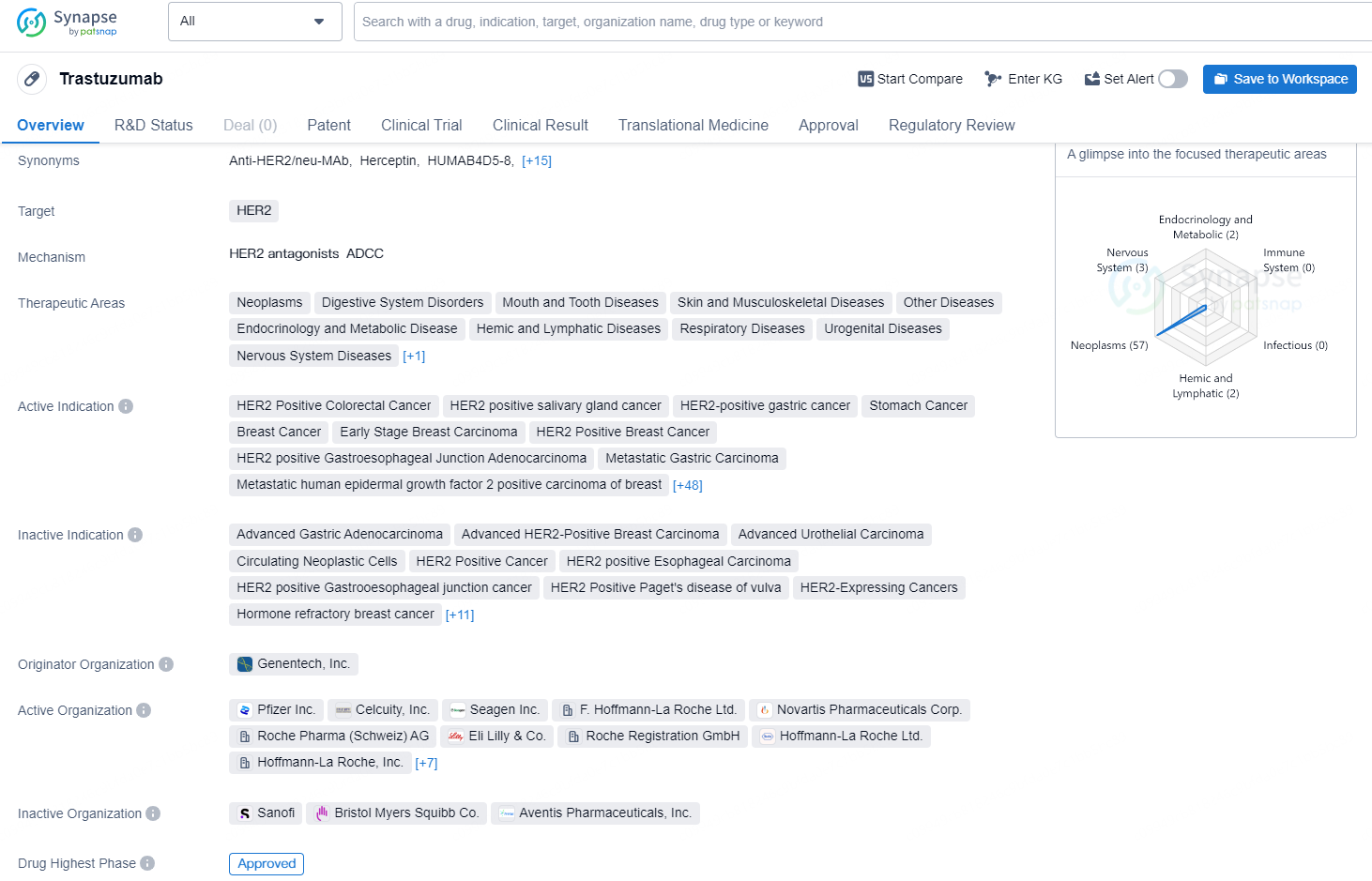
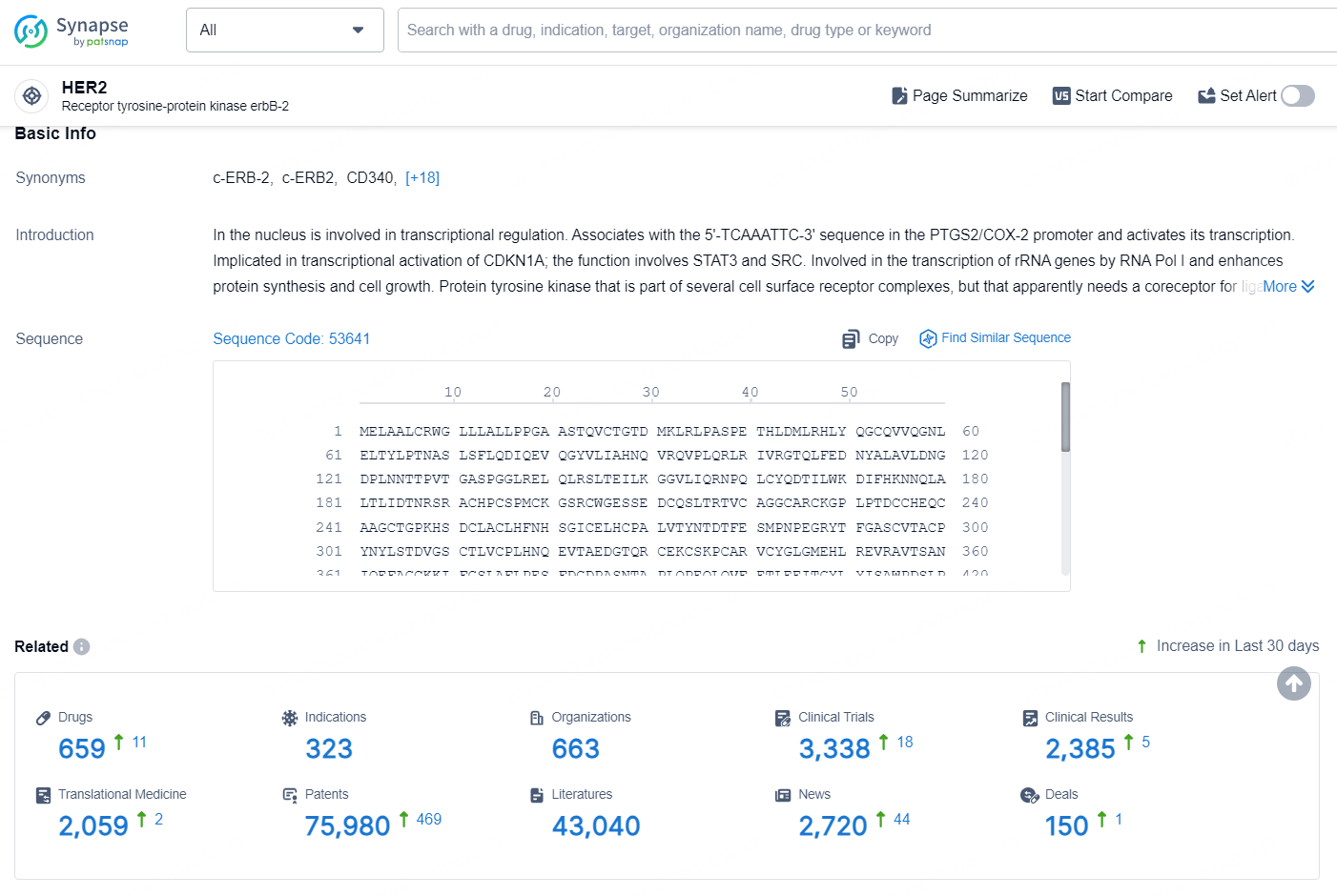
According to the data provided by the Synapse Database, As of September 9, 2024, there are 659 investigational drugs for the HER2 targets, including 323 indications, 663 R&D institutions involved, with related clinical trials reaching 3338, and as many as 75980 patents.
Trastuzumab is a monoclonal antibody drug developed by Genentech, Inc. and was first approved for use in the United States in 1998. The drug targets HER2, a protein that is overexpressed in various types of cancer. Trastuzumab has been approved for the treatment of a wide range of cancers, including HER2 positive breast, colorectal, and gastric cancer, as well as salivary gland, lung, ovarian, and pancreatic cancers, among others.