IGC Pharma Unveils Promising Phase 2 Results for Alzheimer's Drug IGC-AD1
IGC Pharma, Inc. has shared new interim results from its ongoing Phase 2 clinical study assessing IGC-AD1. The primary aim of this trial is to address agitation in Alzheimer's patients, while cognition is evaluated as a secondary outcome. Analysis of the interim data indicates that the group receiving active treatment showed cognitive enhancements compared to the placebo group, representing a significant advancement toward creating a therapy that could potentially impact the progression of the disease.
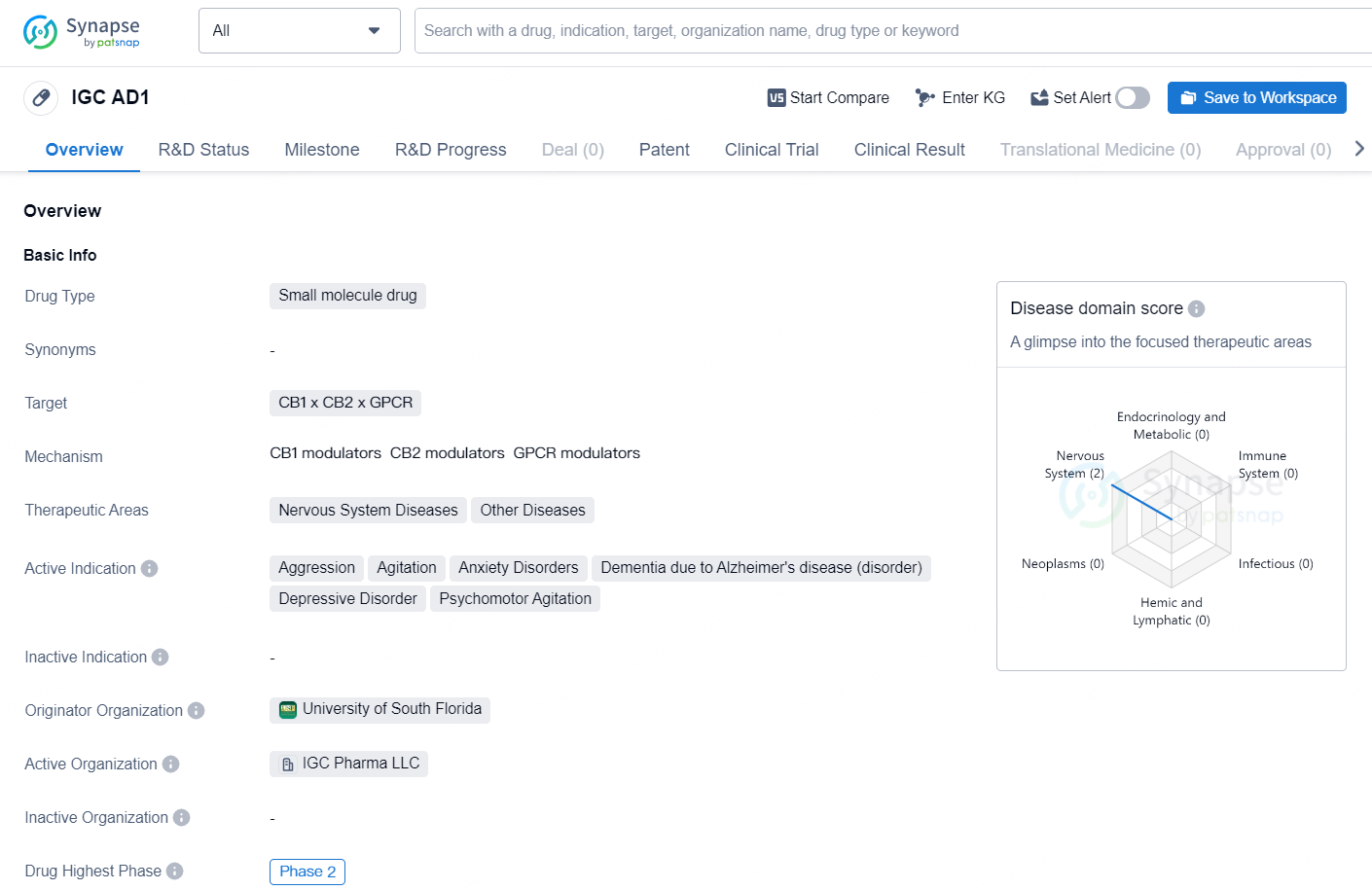
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The results obtained are consistent with earlier preclinical studies that demonstrated a reduction in amyloid plaque aggregation by around 20% and a roughly 50% enhancement in spatial memory within Alzheimer's cell cultures and animal models.
Cognitive Enhancements: A Major Indicator of Disease Advancement
In Alzheimer's disease, cognitive deterioration manifests as a decline in memory, attention, language, and reasoning abilities, resulting from pathological changes such as amyloid plaques and tau tangles. Preliminary findings from the trial indicated that participants in the active treatment group who received IGC-AD1 twice daily for six weeks experienced an average enhancement of approximately 8% on the Mini-Mental State Examination (MMSE), a recognized clinical assessment of cognitive function. In contrast, participants in the placebo group showed no improvement during the same timeframe.
This data, along with the preclinical findings, establishes a solid groundwork for future studies prioritizing cognition as a primary endpoint while investigating the disease-modifying capabilities of IGC-AD1.
Ram Mukunda, CEO of IGC Pharma, remarked, “The cognitive enhancements evident in our interim data are in line with preclinical findings regarding the influence of IGC-AD1’s active pharmaceutical ingredients on amyloid plaques and spatial memory. Although agitation remains the central focus of the Phase 2 trial, these preliminary observations reinforce our belief in IGC-AD1’s potential to tackle the broader aspects of Alzheimer’s disease pathology. For patients and their caregivers, this could signify hope for enhanced treatment, and for our investors, it presents an enticing opportunity within a growing market.”
Principal Objective: Alleviation of Agitation in Alzheimer’s Patients
Agitation is a severe symptom impacting as many as 76% of individuals with Alzheimer's, contributing to enhanced disease progression, increased caregiver stress, and higher hospitalization rates. As mentioned earlier, interim results indicated that IGC-AD1 effectively lowered agitation compared to the placebo, with noticeable benefits observed as early as two weeks post-treatment. Unlike current treatments, which often require 6 to 10 weeks to yield results and carry a black box warning, IGC-AD1 provides swift symptom relief and boasts a favorable safety profile, distinguishing it as a potentially groundbreaking option for managing agitation in Alzheimer's patients.
Alzheimer's disease affects 6.7 million people in the United States alone, with the global market for Alzheimer's treatments expected to surpass $50 billion by 2025. The distinct characteristics of IGC-AD1-offering quick relief for agitation while possibly modifying the disease—set it apart in this rapidly advancing area of healthcare. “These advancements are intended to propel IGC-AD1 toward market availability through further trials and regulatory approvals, potentially revolutionizing patient care and generating significant value for our stakeholders,” Ram Mukunda concluded.
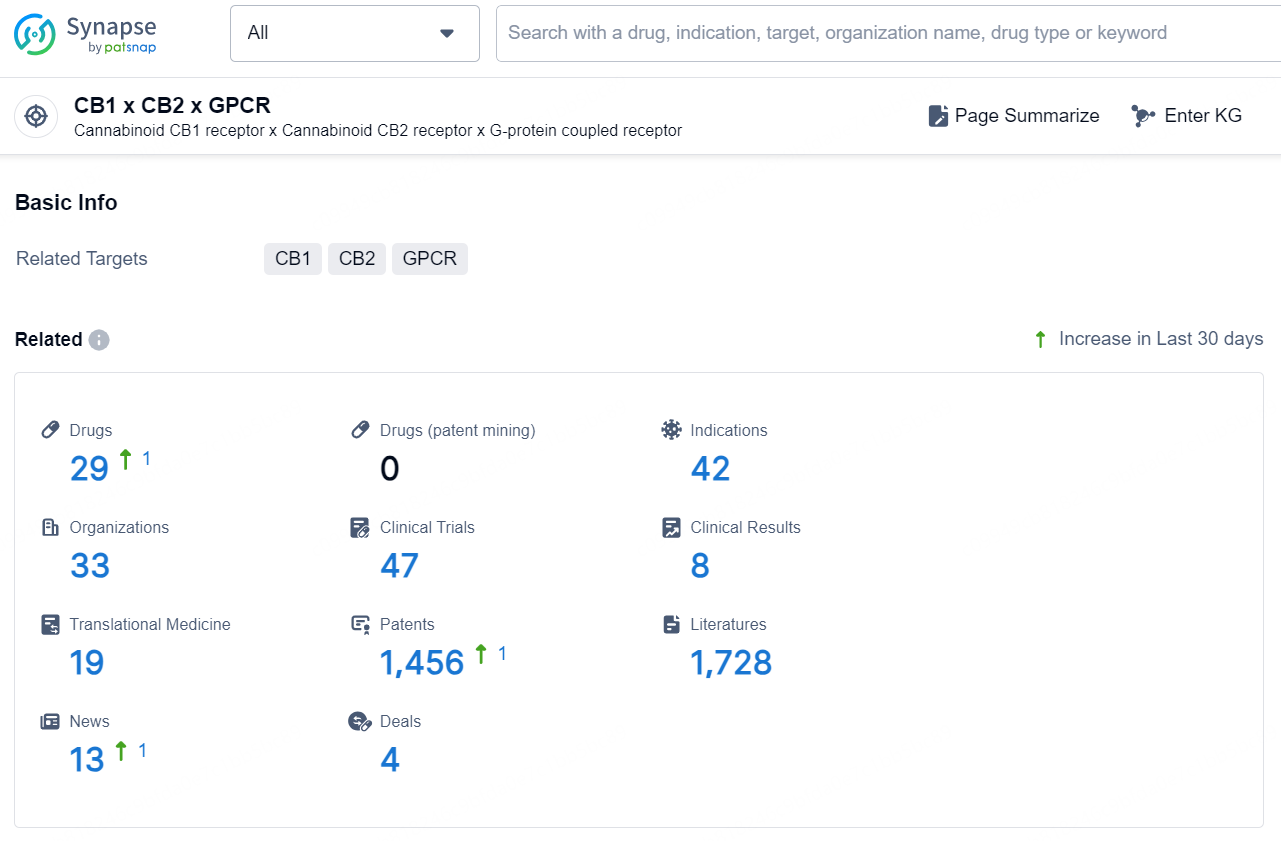
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 2, 2024, there are 29 investigational drugs for the CB1 x CB2 x GPCR target, including 42 indications, 33 R&D institutions involved, with related clinical trials reaching 47, and as many as 1456 patents.
IGC AD1 is a small molecule drug developed by the University of South Florida. The drug targets the CB1 and CB2 receptors as well as the GPCR, making it a potential candidate for treating various nervous system diseases and other disorders. The active indications for IGC AD1 include aggression, agitation, anxiety disorders, dementia due to Alzheimer's disease, depressive disorder, and psychomotor agitation.