Important Research Area in Life Science: Growth Factors
Growth factors represent a class of polypeptides that regulate cell growth and various other cellular functions through binding with specific, high-affinity cell membrane receptors. They are secreted by a variety of cells and act on specific target cells, regulating cell division, matrix synthesis, and tissue differentiation. Growth factors can be found in platelets and various adult and embryonic tissues as well as the majority of cultured cells, exhibiting certain specificity towards different cell types. The growth of cultured cells generally requires the coordination of various growth factors in sequence, while tumor cells exhibit the characteristic of autonomous growth independent of growth factors.
Growth factors are active proteins or polypeptides present in biological bodies that broadly regulate the growth and development of organisms. Their general feature is the ability to bind with specific cell membrane receptors and regulate cell growth and development. They play a major regulatory role in human immunity, hematopoiesis regulation, tumor formation, inflammation and infection, wound healing, angiogenesis, cell differentiation, cell apoptosis, morphogenesis, embryogenesis and other aspects. These cell factors are widely distributed in various types of tissue within the body, including mature and embryonic tissues, and they regulate the proliferation and differentiation of various cells through autocrine and paracrine mechanisms. Many cells cultivated ex vivo can also release growth factors.
In terms of secretion characteristics, growth factors mainly belong to autocrine and paracrine. Many growth factors have been purified and their structural composition has been determined. For instance, the Platelet-Derived Growth Factor (PDGF) is a heat-stable protein with a high positive charge, composed of dimers with disulfide bonds, and has a molecular weight of approximately 30,000 daltons. Similarly, the Epidermal Growth Factor (EGF) is a heat-stable polypeptide containing 53 amino acid residues with a molecular weight of about 6,000 daltons. Every type of growth factor has its corresponding receptor, which are usually transmembrane proteins commonly present on cell membranes. Many of these receptors possess kinase activity, particularly tyrosine kinase activity, such as PDGF and EGF receptors.
The study of cell growth factors is a crucial field in life sciences. The discovery, research, and application of different growth factors play a significant role in advancing the development of life sciences. The study of cell growth factors not only explains the regulatory control in biological growth and development and other physiological functions, but also enables humans to better understand the occurrence of diseases, tissue injury, and pathological changes in repair, enhancing human knowledge about disease prevention, diagnosis, treatment, and recovery, while providing innovative strategies.
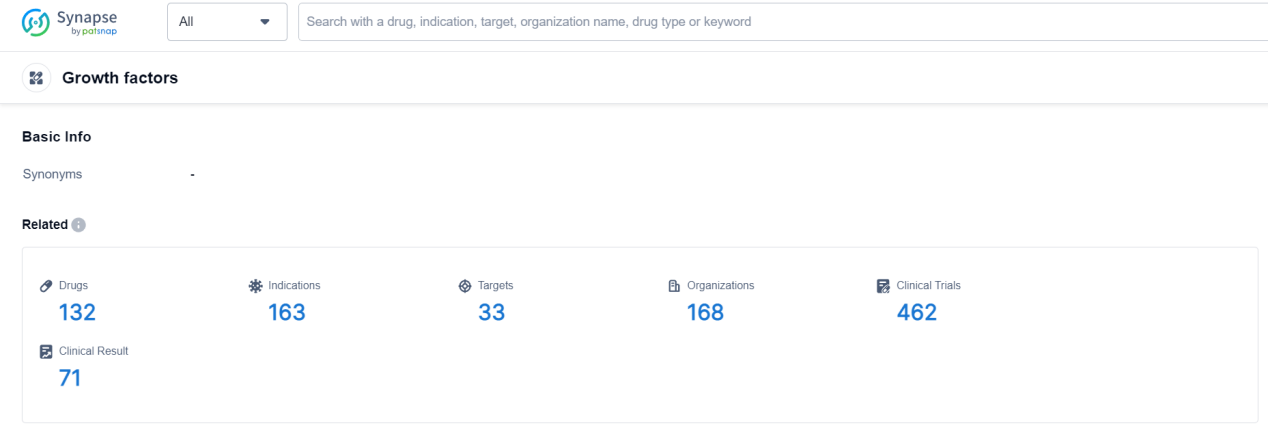
Growth Factors Competitive Landscape
According to Patsnap Synapse, as of 19 Sep 2023, there are a total of 132 Growth factors drugs worldwide, from 168 organizations, covering 33 targets, 163 indications, and conducting 462 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this drug type.
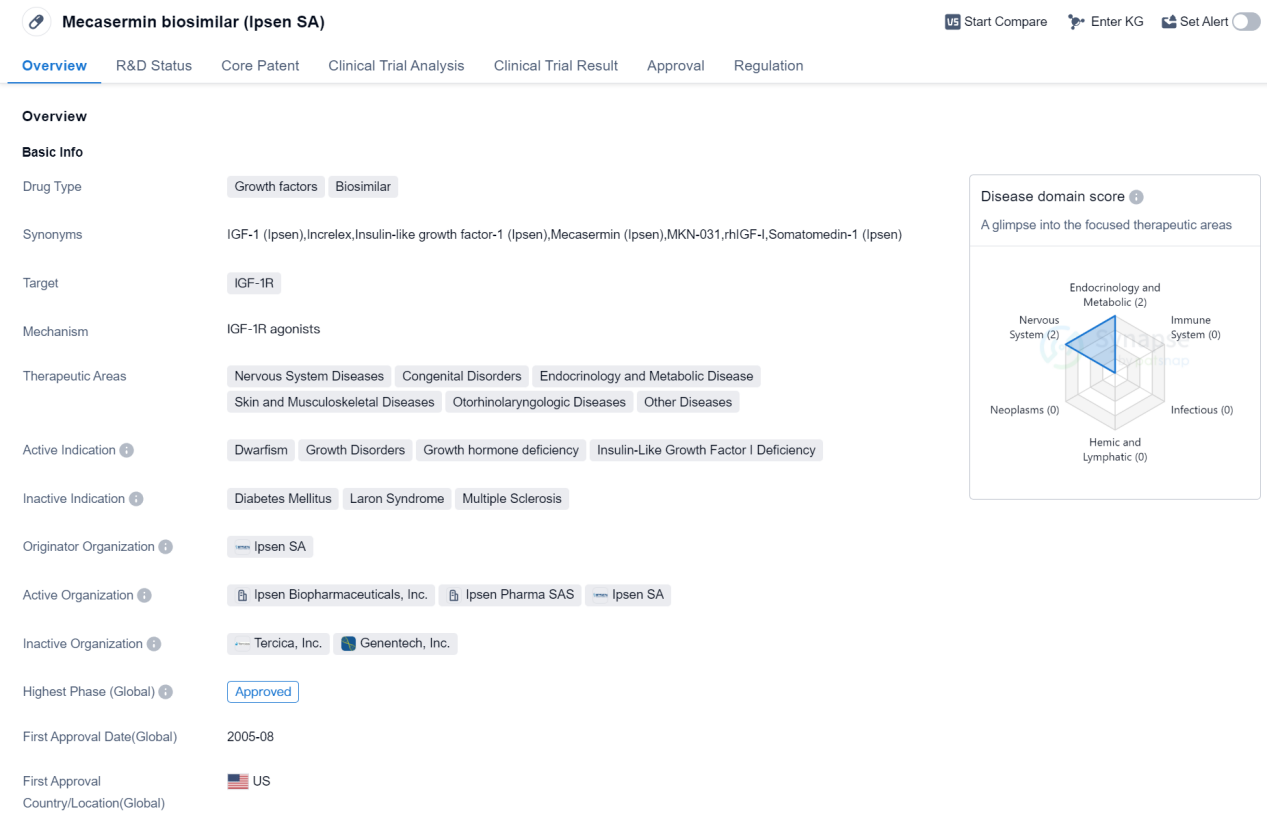
Approved Growth Factors Medicinal: Mecasermin biosimilar
Mecasermin biosimilar, developed by Ipsen SA, is a growth factor drug that acts as a biosimilar to the insulin-like growth factor 1 receptor (IGF-1R). It has been approved for use in treating various therapeutic areas, including nervous system diseases, congenital disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, otorhinolaryngologic diseases, and other diseases.
The drug is primarily indicated for the treatment of dwarfism, growth disorders, growth hormone deficiency, and insulin-like growth factor 1 deficiency. It works by stimulating the IGF-1R, which plays a crucial role in promoting growth and development in the body.
Mecasermin biosimilar received its first approval in the United States in August 2005. It is classified as an overseas new drug urgently needed in clinical settings, indicating its importance in addressing unmet medical needs. This classification suggests that the drug may have been fast-tracked through the regulatory process due to its potential benefits and urgency in treating certain conditions.
As a biosimilar, Mecasermin is developed as a highly similar version of the original drug, which in this case is also developed by Ipsen SA. Biosimilars are designed to have comparable efficacy, safety, and quality to the reference product, providing an alternative treatment option at a potentially lower cost.
The approval of Mecasermin biosimilar in multiple therapeutic areas highlights its versatility and potential to address a wide range of medical conditions. By targeting the IGF-1R, the drug offers a targeted approach to treating growth-related disorders and deficiencies.
Overall, Mecasermin biosimilar represents an important advancement in the field of biomedicine, particularly in the treatment of growth-related conditions. Its approval in various therapeutic areas and its classification as an urgently needed drug demonstrate its potential to improve patient outcomes and address unmet medical needs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
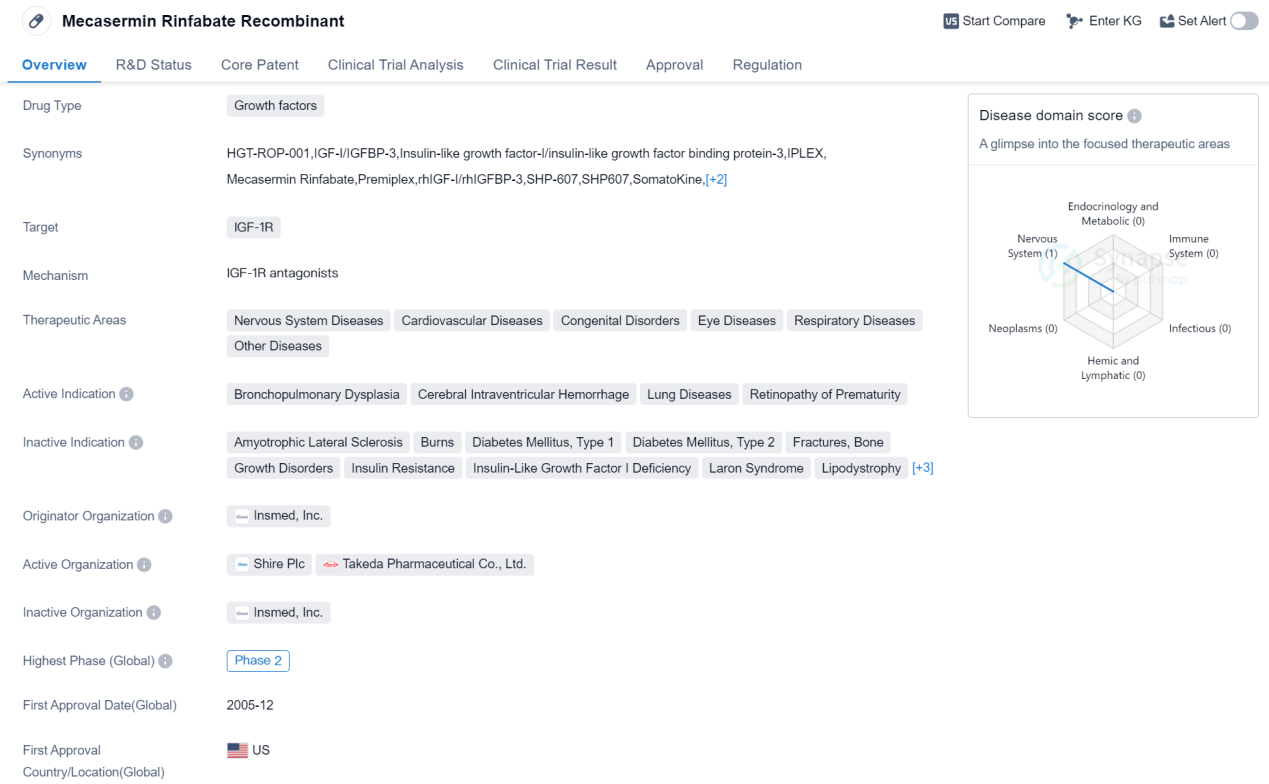
Phase II Clinical Trial Of Growth Factors Medicinal: Mecasermin Rinfabate Recombinant
Mecasermin Rinfabate Recombinant is a drug classified as a growth factor and it targets the IGF-1R (insulin-like growth factor-1 receptor). It has been developed by Insmed, Inc., an originator organization in the pharmaceutical industry. The drug has received its first approval in the United States in December 2005 and is regulated as an orphan drug.
The therapeutic areas in which Mecasermin Rinfabate Recombinant is indicated include Nervous System Diseases, Cardiovascular Diseases, Congenital Disorders, Eye Diseases, Respiratory Diseases, and Other Diseases. Specifically, it is used to treat conditions such as Bronchopulmonary Dysplasia, Cerebral Intraventricular Hemorrhage, Lung Diseases, and Retinopathy of Prematurity.
In terms of its development stage, Mecasermin Rinfabate Recombinant has reached the highest phase of Phase 2 globally. However, its highest phase in China is still pending, indicating that it is yet to progress through the clinical trial phases in that country.
The drug's classification as an orphan drug suggests that it is intended to treat rare diseases or conditions that affect a small number of patients. Orphan drugs often receive special regulatory incentives to encourage their development and availability.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Overall, Mecasermin Rinfabate Recombinant is a growth factor drug that targets the IGF-1R and has been approved for use in the United States. Its therapeutic areas cover a range of diseases affecting the nervous system, cardiovascular system, congenital disorders, eye diseases, respiratory system, and other conditions. While it has reached Phase 2 in global development, its progress in China is still pending. The drug's orphan drug status indicates its focus on treating rare diseases, and it was first approved in December 2005.