IMUNON Announces Data from Phase 1/2 OVATION 2 Trial for Late-Stage Ovarian Cancer
IMUNON, Inc., a biotech firm in the clinical phase that concentrates on the advancement of DNA-driven immunotherapies and next-gen vaccines, released preliminary PFS and OS statistics related to IMNN-001 from its Phase 1/2 OVATION 2 Investigation. The complete recruitment of 110 participants was accomplished in September 2022.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
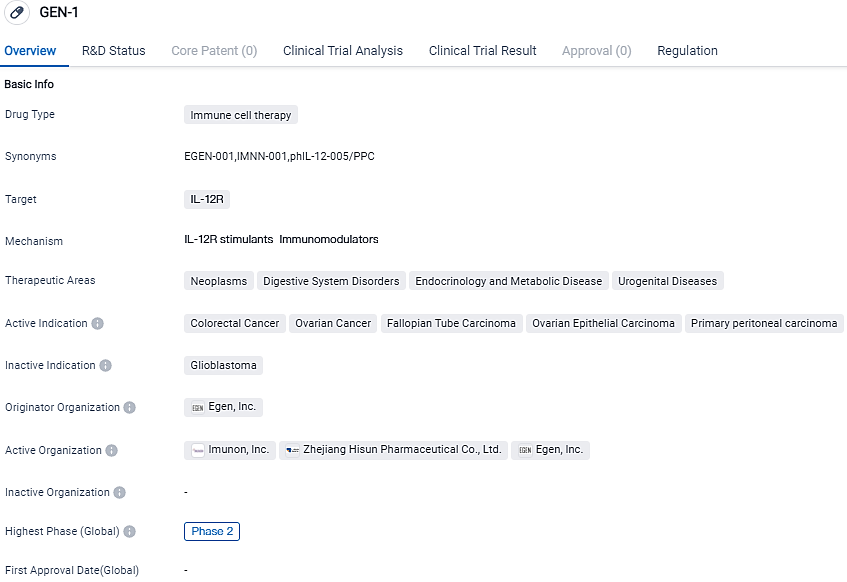
OVATION 2 aims to assess the dosage, safety, impact and biological action of IMNN-001, used intraperitoneally with neoadjuvant chemotherapy, in discovering patients with advanced epithelial ovarian, fallopian tube and primary peritoneal cancer. Neoadjuvant chemotherapy intends to diminish the size of tumors to facilitate optimal surgery post three cycles of chemotherapy. Following NACT, patients undergo interval debulking surgery, followed by extra chemotherapy cycles to address any residual tumor.
As expected for a Phase 1/2 study, the study is directional and was set with an 80% confidence interval to exhibit around 33% improvement in PFS while comparing the therapy group with the control group. Secondary outcomes involve OS, ORR, pathological response, surgical response, and serologic response. Study final readouts are projected to be available by mid-2024. A positive readout would signal future development phases.
Furthermore, the preliminary data from the intent-to-treat population that was unveiled today suggests positive trends in PFS, showcasing a delay in disease progression by around 33% in the treatment group when put against the control group, with the hazard ratio approaching the necessary value. Preparatory OS data also follows a comparable path, revealing an estimated 9-month improvement in the treatment group versus the control group.
Dr. Corinne Le Goff, President & CEO of IMUNON, commented on these initial findings, stating, "These preliminary results reassures us, especially the overall survival trends in patients who were administered PARP inhibitors, neoadjuvant chemotherapy, and IMNN-001. Even though the patient count in this subgroup is relatively limited, this routine might offer potential treatment methods as we continue tracking patients participating in OVATION 2, expecting to announce the primary results by mid-2024."
Built with IMUNON's exclusive TheraPlas platform technology, IMNN-001 is an IL-12 DNA plasmid vector housed within a nanoparticle distribution method, allowing cellular transfection followed by persistent local secretion of the IL-12 protein. IL-12 is one of the most active cytokines initiating robust anticancer immunity through promoting T-lymphocyte and natural killer cell proliferation.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
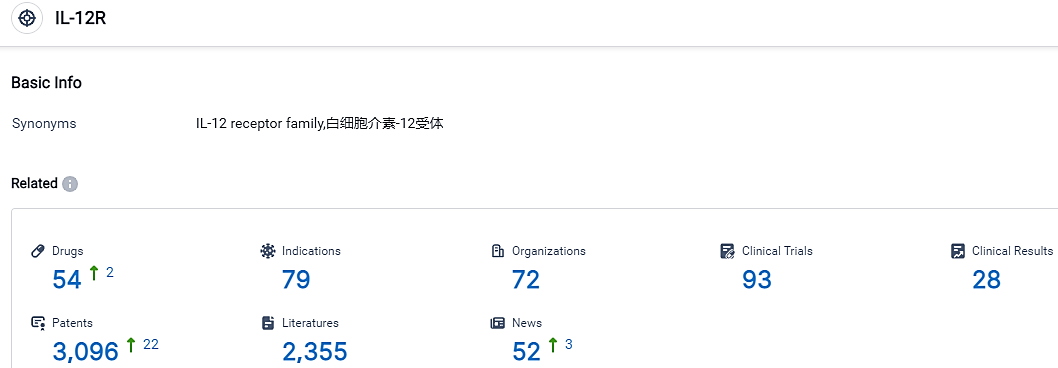
According to the data provided by the Synapse Database, As of October 9, 2023, there are 54 investigational drugs for the IL-12R target, including 79 indications, 72 R&D institutions involved, with related clinical trials reaching 93,and as many as 3096 patents.
GEN-1 is an immune cell therapy drug that specifically targets the IL-12R receptor. With its current highest phase of development being Phase 2, both globally and in China, GEN-1 has shown promising results in early clinical trials. Additionally, the drug has been granted Fast Track status, indicating its potential to address unmet medical needs and expedite its development and review.