INCB-057643: brief review of its R&D progress and the clinical result in 2023 ASH
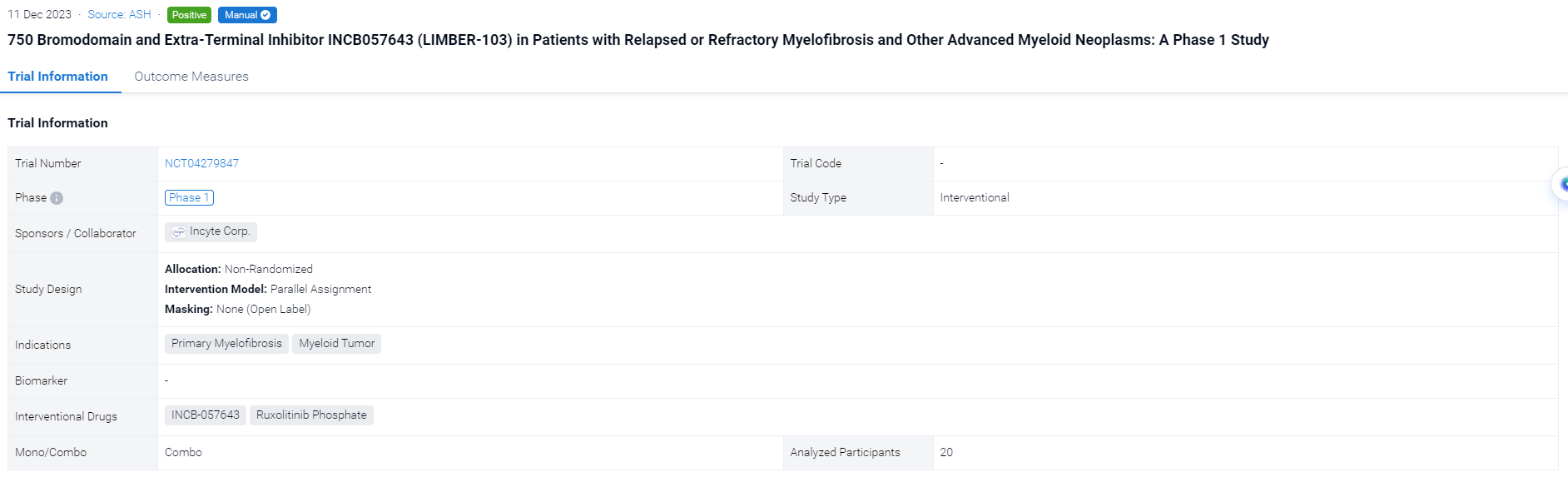
On December 11, 2023, This ongoing phase 1, 3+3 dose-escalation/expansion study (NCT04279847) to evaluate the safety and tolerability of INCB057643 will be reported at the ASH Congress.
INCB-057643's R&D Progress
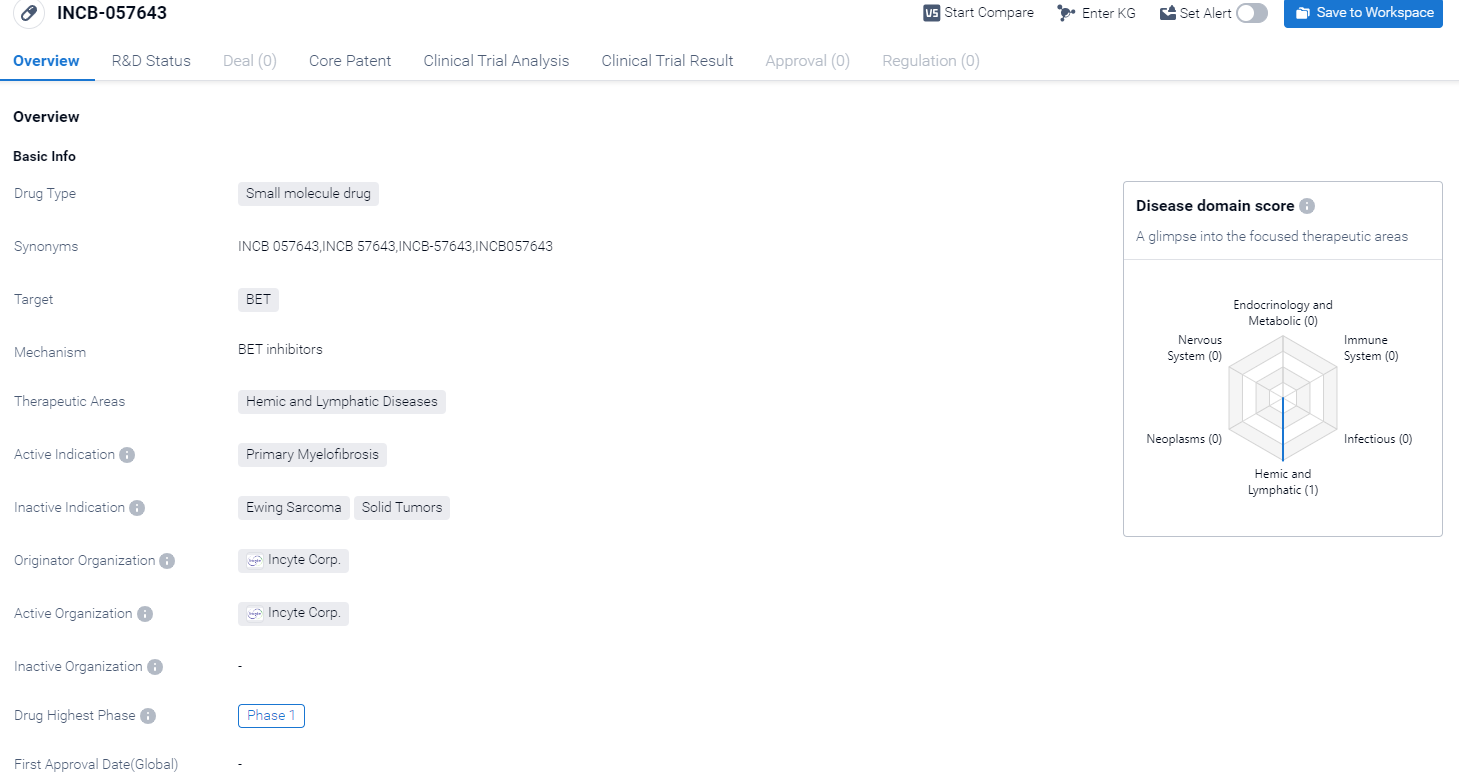
INCB-057643 is a small molecule drug that falls under the therapeutic area of Hemic and Lymphatic Diseases. It specifically targets BET proteins, which are involved in the regulation of gene expression. The drug is being developed for the treatment of Primary Myelofibrosis, a rare and chronic blood disorder characterized by the scarring of bone marrow and the overproduction of fibrous tissue.
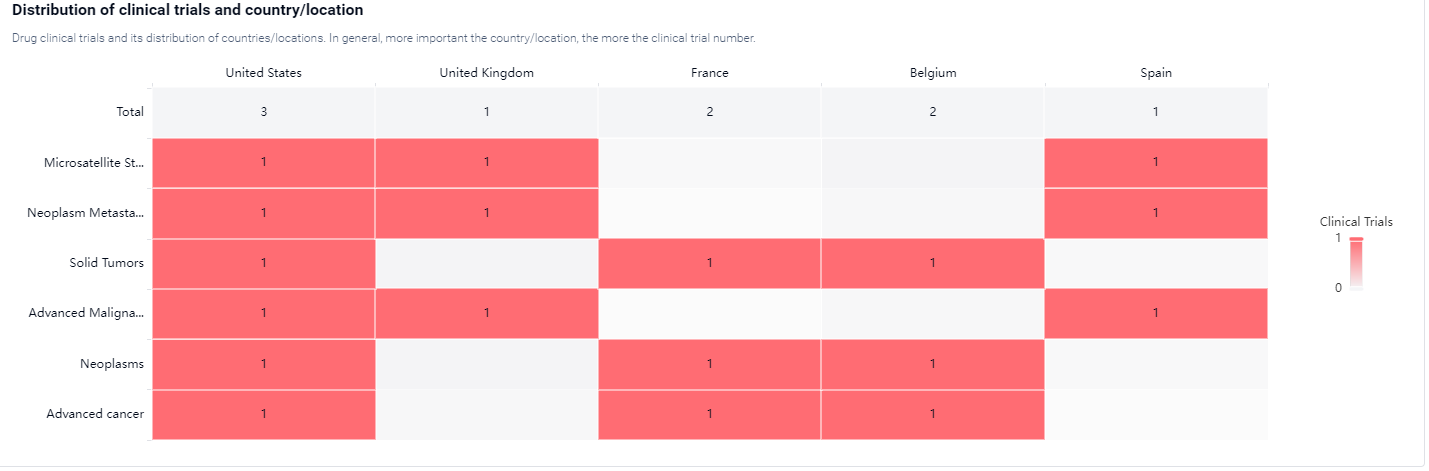
According to the Patsnap Synapse, INCB-057643 is currently in the highest phase of clinical development, which is Phase 1. And the clinical trial distribution for INCB-057643 are primarily in the United States, United Kingdom, France, Belgium and Spain. The key indication is Microsatellite Stable Colorectal Carcinoma, Neoplasm Metastasis, solid Tumors, etc.
Detailed Clinical Results of INCB-057643
The non-randomized, parallel assignment, open-labeled clinical trial (NCT04279847) was conducted in patients (pts) with relapsed or refractory myelofibrosis (R/R MF) and other advanced myeloid neoplasms.
In this study, INCB057643 (4 mg once daily [qd]; escalation up to 12 mg qd) was used in pts aged ≥18 years as (1) monotherapy (part 1) in R/R MF, myelodysplastic syndromes (MDS), or MDS/myeloproliferative neoplasm (MPN) overlap syndromes (MDS/MPN) or (2) added to RUX (part 2) in pts with MF and suboptimal response to RUX.
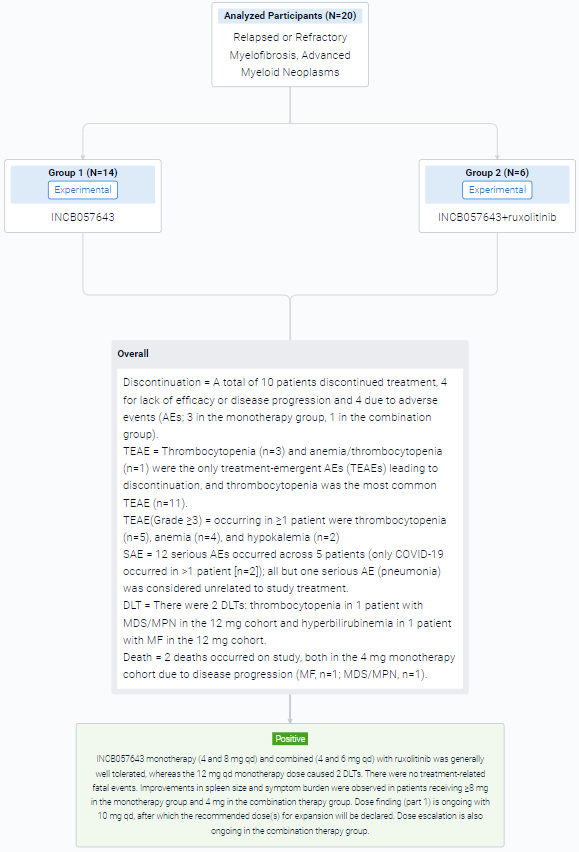
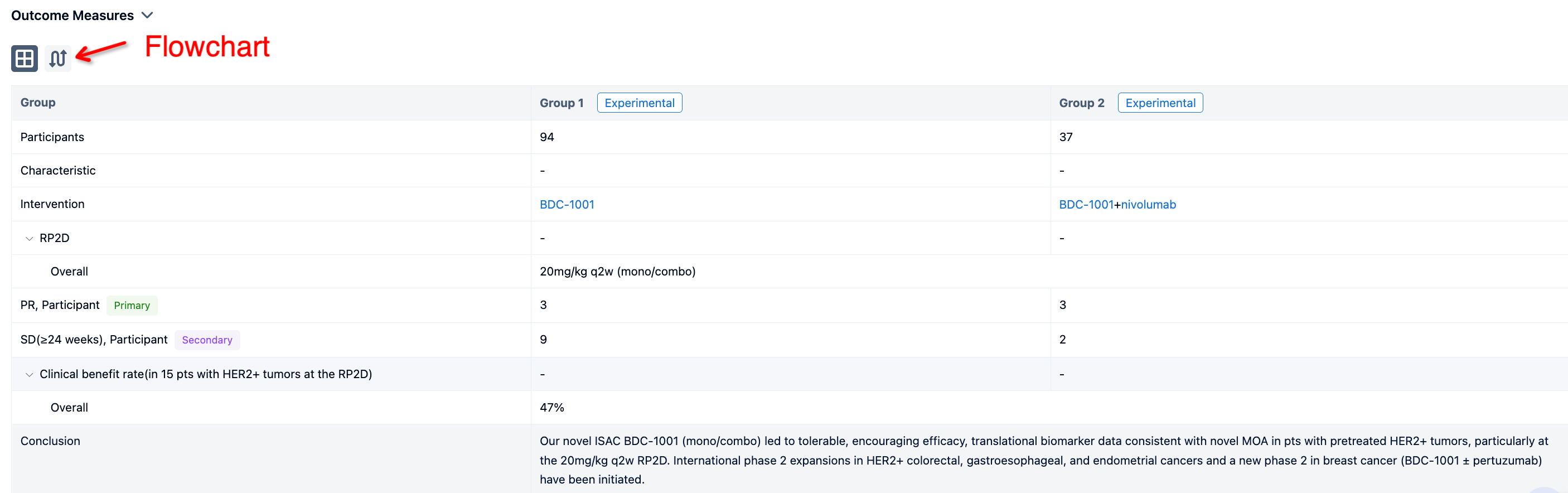
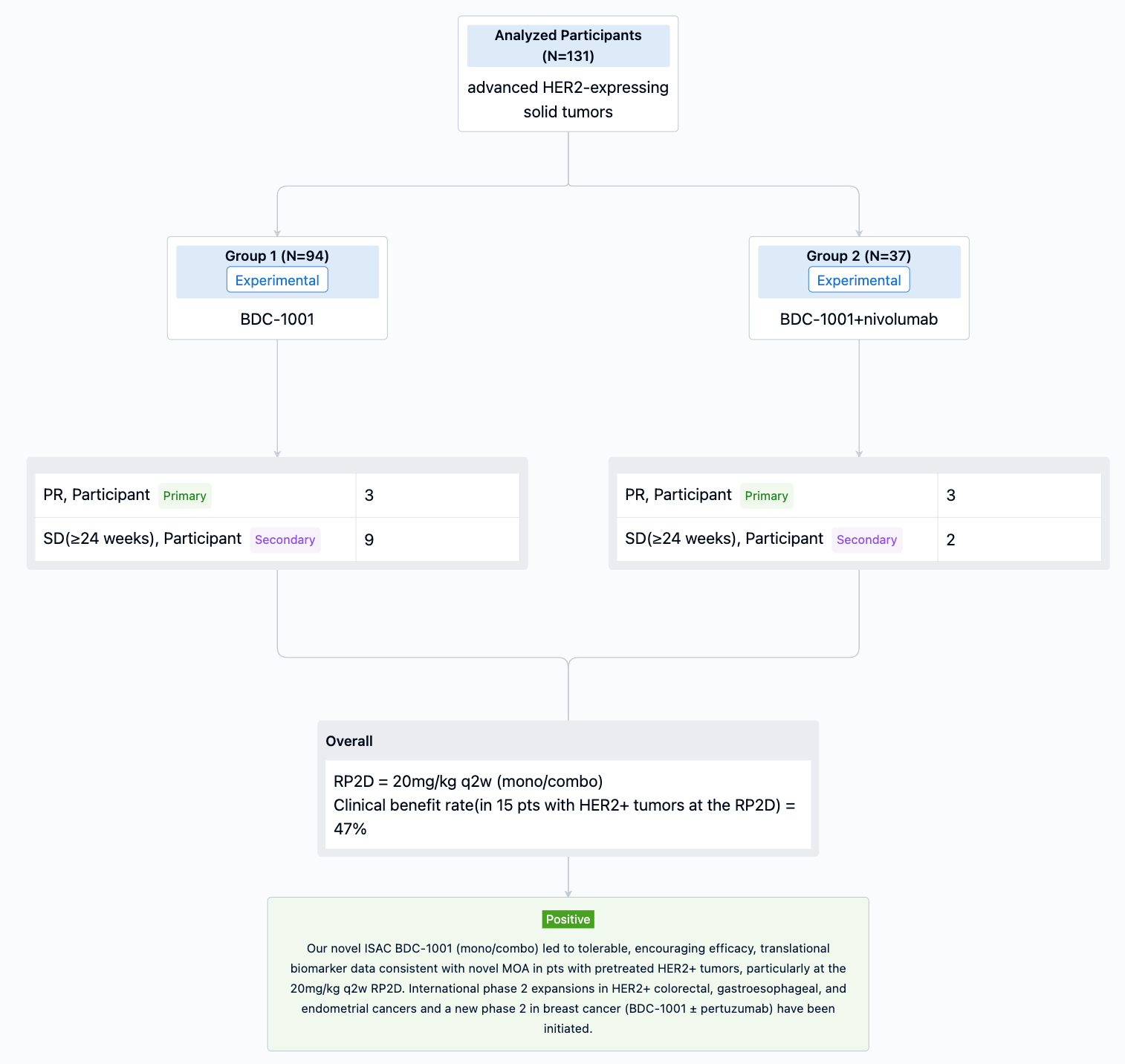
The result showed that 13 pts have been treated in part 1 (4 mg, n=6; 8 mg, n=4; 10 mg, n=1; 12 mg, n=2), and 3 pts received 4 mg + RUX in part 2 (overall age range, 50–79 years; men, n=9; study treatment duration range, 15–314 days). 12 pts had MF, and 4 had MDS/MPN. All 6 pts in the 4-mg cohort discontinued treatment (3 for progressive disease [PD]; MF, n=2; MDS/MPN, n=1); 1 pt with MF in the 12-mg cohort discontinued for thrombocytopenia. The other 9 pts remain on treatment. Thrombocytopenia was the most common treatment-emergent adverse event (TEAE; n=9; Table) and the only TEAE leading to discontinuation (n=3). Grade ≥3 TEAEs occurring in ≥1 pt were thrombocytopenia (n=4), anemia (n=3), and hypokalemia (n=2). There were 8 serious AEs across 4 pts, with only COVID-19 occurring in >1 pt (n=2); all but one (pneumonia) were considered unrelated to study treatment. There were 2 DLTs (thrombocytopenia [MDS/MPN pt] and hyperbilirubinemia [MF pt]; both 12-mg cohort) and 2 deaths (both 4-mg cohort due to PD [MF, n=1; MDS/MPN, n=1]).
It can be concluded that treatment with INCB057643 monotherapy (4 and 8 mg qd) and in combination (4 mg qd) with RUX was generally well tolerated in this pt population. The 12-mg qd monotherapy dose was not tolerated and caused 2 DLTs. There were no treatment-related fatal events. Dose finding in part 1 is ongoing with 10 mg qd, after which a recommended phase 2 dose will be declared. Combination dose escalation is also ongoing. Preliminary efficacy including spleen size and symptoms will be available for presentation.
How to Easily View the Clinical Results Using Synapse Database?
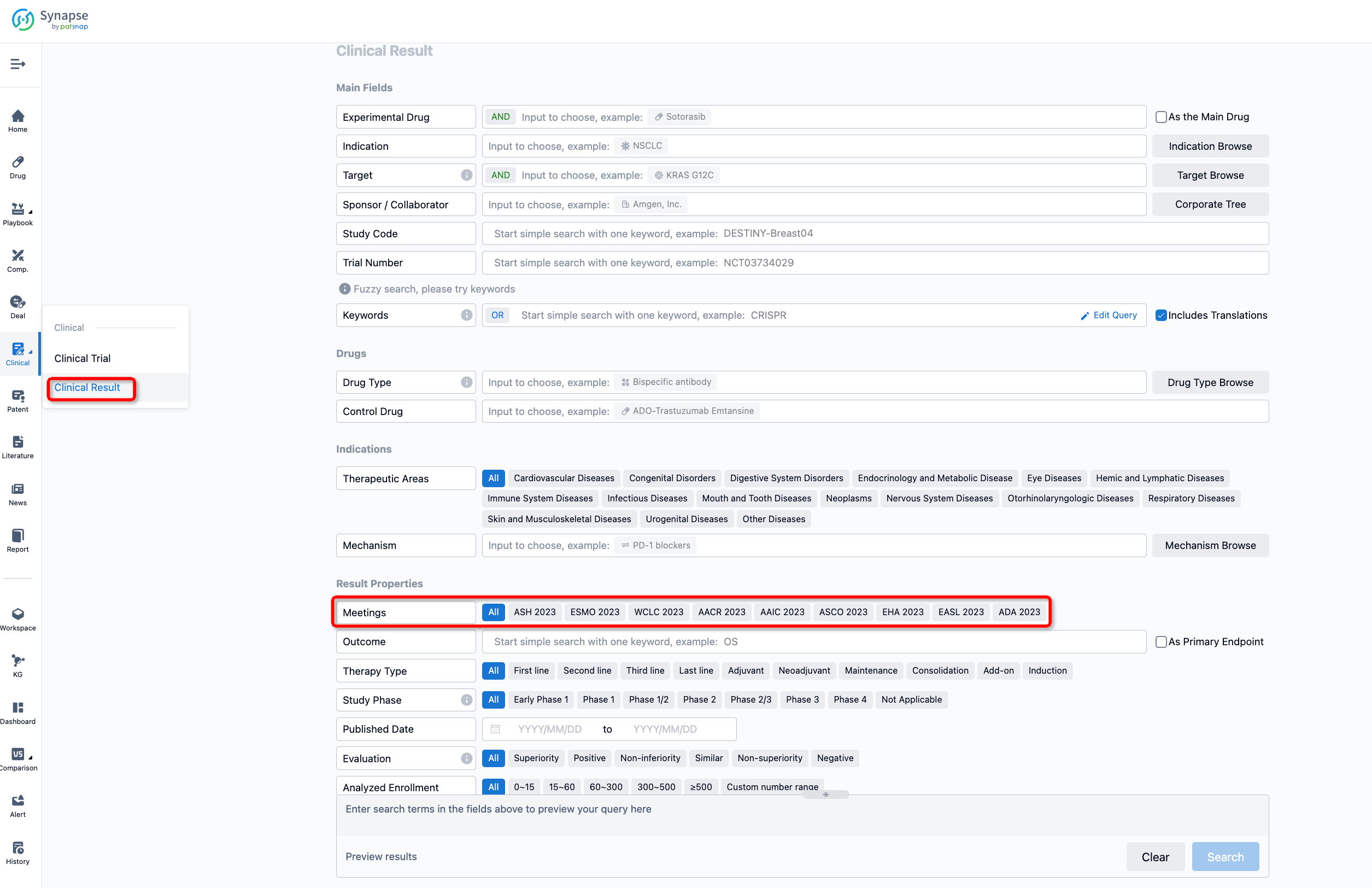
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
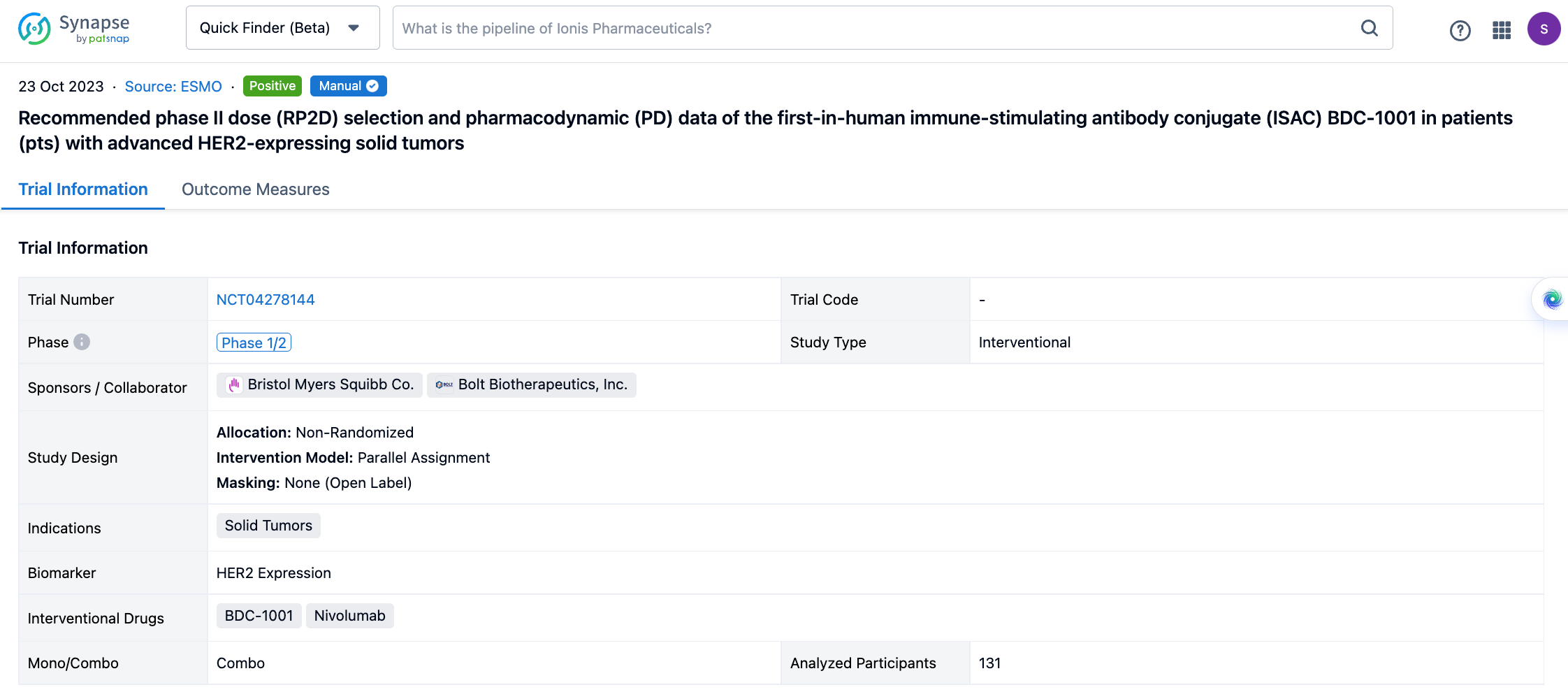
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
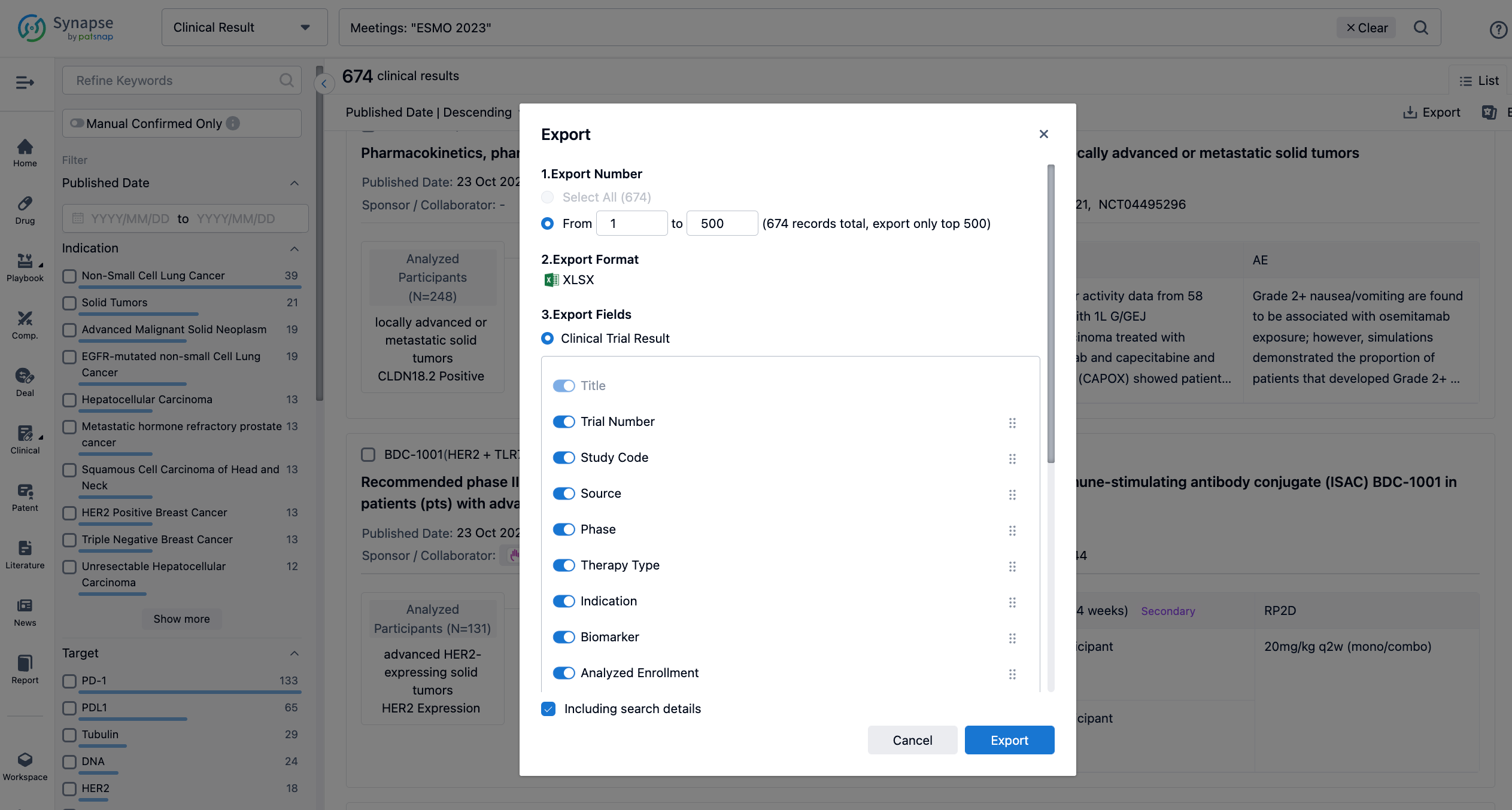
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!