Initial Human Participant Receives ZMA001 in Medical Study Targeting PAH Therapy
Zymedi has initiated the maiden human administration of ZMA001, a pioneering monoclonal antibody crafted to treat Pulmonary Arterial Hypertension. This groundbreaking step marks the beginning of a new potential therapy option for this medical condition.
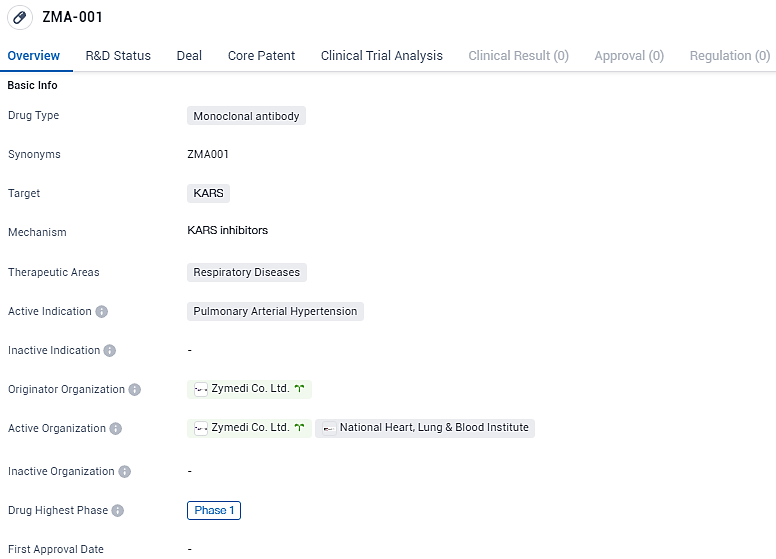
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In a concerted endeavor with the National Heart, Lung, and Blood Institute, a component of the National Institutes of Health, Zymedi has initiated a phase 1 study with a placebo comparison to evaluate the tolerability and appropriate levels of administration for the drug ZMA001. This undertaking follows the previous year's formation of a Cooperative Research & Development Agreement between the two institutions aimed at propelling the progress of ZMA001.
Pulmonary Arterial Hypertension (PAH) poses a severe and potentially fatal threat affecting close to 3 individuals out of every 100,000 across the globe, with an estimated range of 500 to 1,000 people receiving a diagnosis each year within the United States alone. PAH is distinguished by the constriction of the pulmonary arteries, an increase in pulmonary arterial tension, and ultimately, fatality due to the failure of the right side of the heart.
Despite the availability of current treatments such as vasodilators, which offer symptom relief, they fall short of addressing the foundational issue. ZMA001 emerges as a pioneering therapeutic antibody aimed at the recently identified inflammatory agent KARS1, which predominantly affects monocytes and macrophages. Its ability extends to enhancing patient survival probabilities and life quality by directly attacking the fundamental pathological factor responsible for PAH.
Dr. Sunghoon Kim, the CEO and founder of Zymedi, expressed his enthusiasm about launching this novel human study of ZMA001, recognizing it as an important progression for his company and the ongoing battle against PAH. Dr. Kim disclosed the company's plans to proceed with the critical evaluation of ZMA001's safety and tolerability by gradually increasing dosages in subsequent dose-escalation studies, carefully monitoring for any adverse effects correlated with the dosing increments.
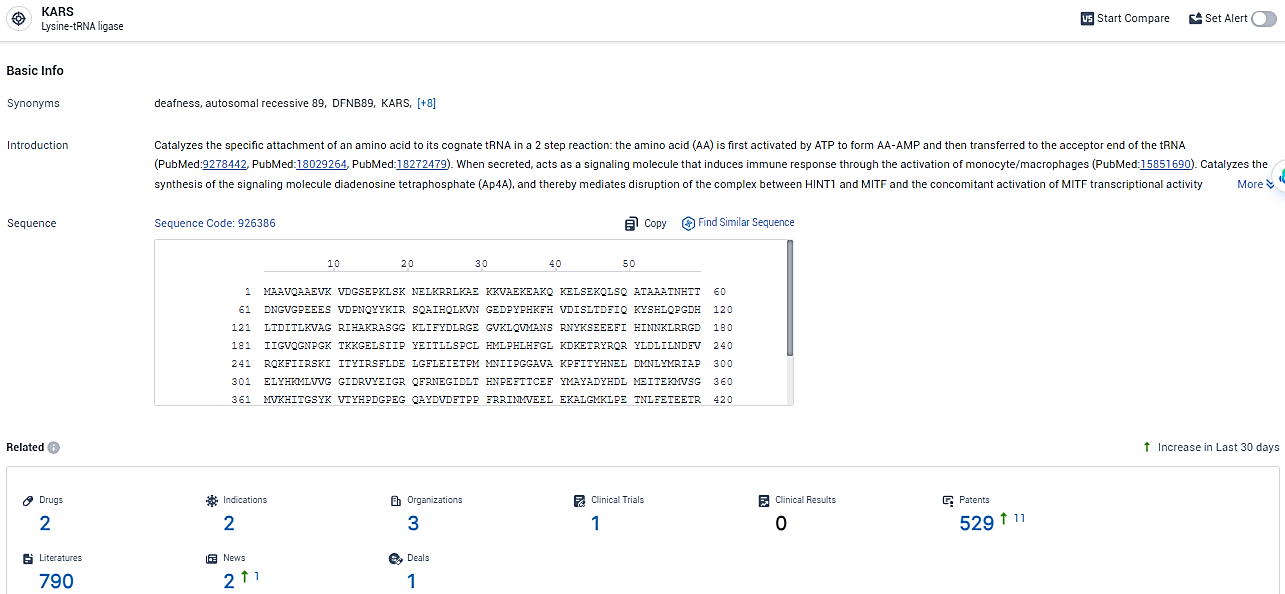
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 19, 2024, there are 2 investigational drugs for the KARS target, including 2 indications, 3 R&D institutions involved, with related clinical trials reaching 1, and as many as 529 patents.
ZMA001 specifically targets membrane-exposed KARS1 without affecting its catalytic activity for protein synthesis. Currently in Phase 1 of development, ZMA-001 shows promise as a potential therapeutic option for patients with this respiratory disease. Further research and clinical trials will be necessary to determine its safety and efficacy in larger patient populations.