Initial Participant Receives First Treatment in Henlius' HLX42 ADC Early-Stage Study
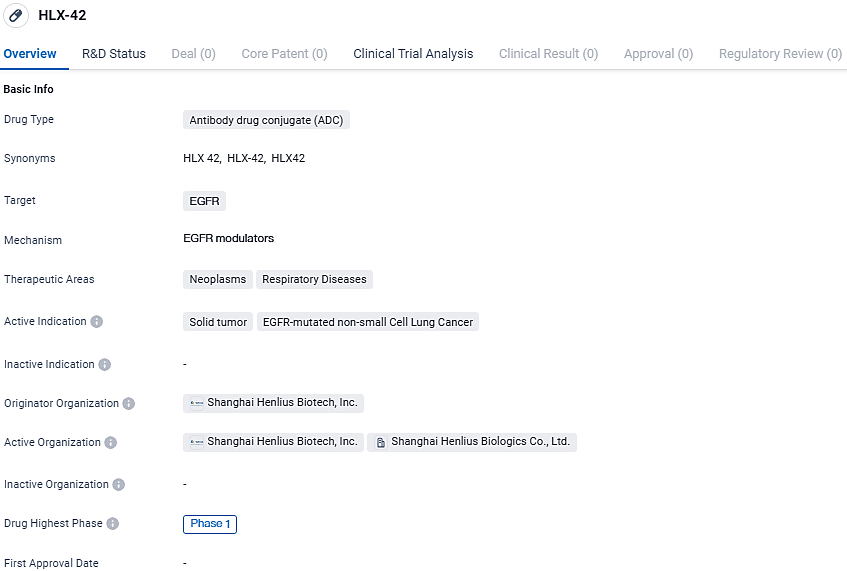
Shanghai Henlius Biotech, Inc. has reported the commencement of a phase 1 clinical study for HLX42, by administering the initial dose to a participant. HLX42 is a novel antibody-drug conjugate directed at EGFR, currently under investigation for its efficacy in managing advanced or metastatic solid tumors.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The pharmaceutical entity HLX42, a product of the joint venture between MediLink Therapeutics and the initiating company, has received authorization from both the National Medical Products Administration and the U.S. Food and Drug Administration to embark on clinical trials.
By the close of December 2023, the U.S. FDA had acknowledged the significance of HLX42 by bestowing upon it the Fast Track Designation for its role in managing patients struggling with advanced or metastatic non-small cell lung cancer who have not seen improvement with EGFR targeted treatments.
Despite impressive strides made with monoclonal antibodies against EGFR and third-generation EGFR Tyrosine kinase inhibitors, research indicates that new treatments are imperative for patients who demonstrate resistance or relapse following conventional therapy regimens.
HLX42 is engineered as an innovative ADC targeting EGFR. Its architecture includes a humanised IgG1 monoclonal antibody with high affinity for EGFR, linked to a groundbreaking toxic agent via cleavable connections, and it boasts a drug-to-antibody ratio of approximately 8.
Through its robust anti-tumour efficacy demonstrated in various CDX and PDX models resistant to established EGFR TKIs or cetuximab, HLX42 promises to address the challenge of resistance to current EGFR targeting treatments, thereby serving the significant therapeutic requirements of a broader patient group afflicted with advanced or metastatic solid tumours.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
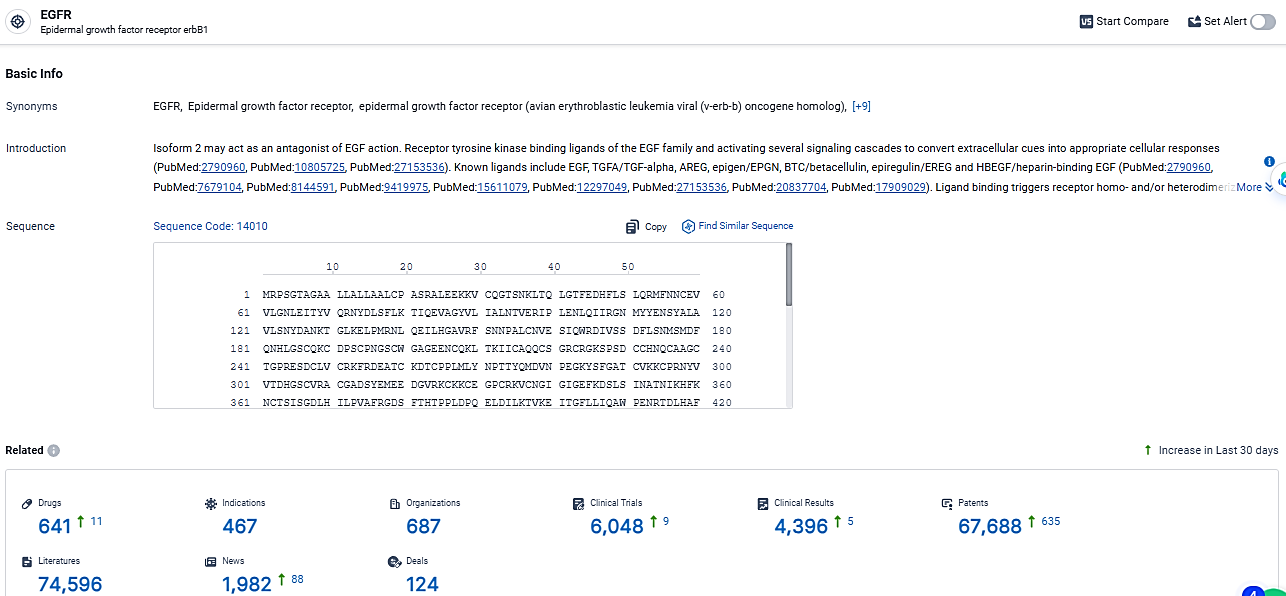
According to the data provided by the Synapse Database, As of March 19, 2024, there are 641 investigational drugs for the EGFR target, including 467 indications, 687 R&D institutions involved, with related clinical trials reaching 6048, and as many as 67688 patents.
HLX-42 targets the EGFR and aims to treat solid tumors, specifically EGFR-mutated non-small cell lung cancer. The drug is currently in Phase 1 of clinical development both globally and in China. Further research and development will be needed to assess the safety and efficacy of HLX-42 in larger patient populations and to progress to later stages of clinical testing.