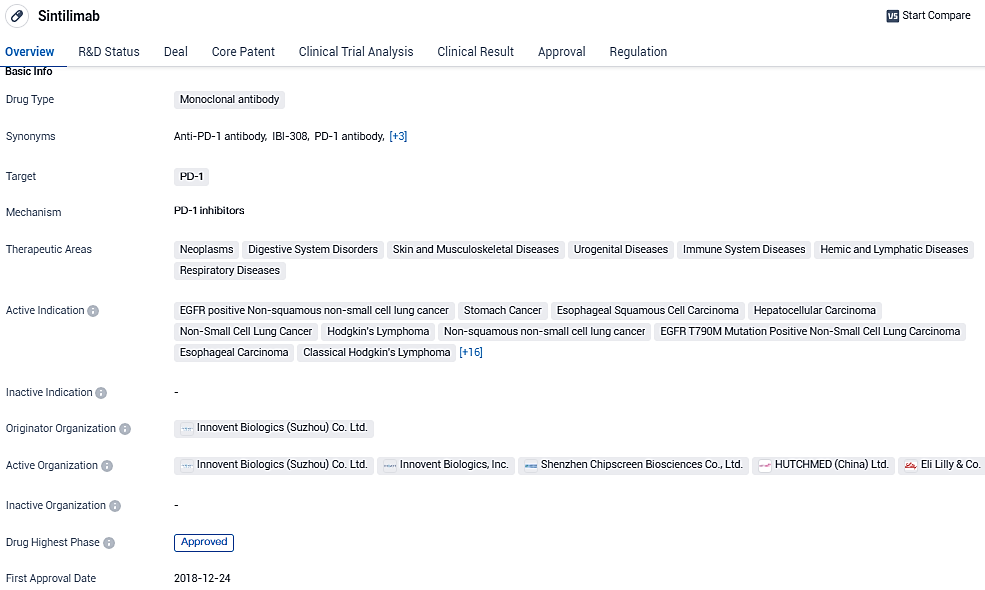
Innovent, Xuanzhu Launch Study on Sintilimab (PD-1 Inhibitor) and New ADC for Advanced Solid Tumors in China
Innovent Biologics, Inc., in partnership with Xuanzhu Biopharma, has publicly disclosed the initiation of a cooperative clinical trial and provision arrangement concerning the synergistic use of the medication sintilimab (marketed as TYVYT®) alongside KM-501, an innovative HER-2 targeted bispecific antibody-drug conjugate (ADC). This collaborative effort is aimed at exploring the efficacy of these combined therapies in addressing complex solid tumors within the Chinese medical landscape.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As per the contract, Innovent is tasked with providing sintilimab for the joint clinical study. Xuanzhu Biopharma is set to carry out an initial Phase 1b clinical trial with the aim of assessing both the efficacy in combatting tumors and the safety profile of a co-therapy regimen consisting of sintilimab and KM-501 in a group of Chinese patients suffering from advanced solid tumors.
The compound TYVYT® (sintilimab injection) represents a groundbreaking inhibitor of PD-1, resulting from a joint development effort between Innovent and Eli Lilly and Company within the Chinese market. It has earned approval and has been incorporated into the national reimbursement drug list, covering a total of seven different uses. Notably, it is the exclusive PD-1 inhibitor indicated for primary line treatment in the case of five types of cancer with substantial incidence figures, as specified in the NRDL.
KM-501 has been developed using the Mebs-Ig platform that holds proprietary intellectual property rights and is an innovative dual-antibody ADC targeting two distinct HER2 domains. It has been deemed appropriate for combating advanced solid tumors, including metastatic growths, characterized by HER2 positivity/presence, amplified levels, or mutations. Its spectrum also extends to encompass advanced tumors that demonstrate low HER2 expression. The IND for this drug was sanctioned by the NMPA in March of the current year, and it is at present undergoing a Phase I dose-escalation research phase.
The combined use of a PD-1 inhibitor along with an ADC drug candidate is emerging as an exciting strategy in the field of cancer treatment, with potential implications for enhancing immunotherapy outcomes and addressing issues of drug resistance. By integrating PD-1-based immunotherapy, which can restore the reactivity of T cells, with an ADC that targets tumor cells with cytotoxic agents, the approach could foster a complementary effect that inhibits tumor growth more effectively.
Dr. Hui Zhou, who serves as a Senior Vice President at Innovent, remarked: "Engaging in this partnership with Xuanzhu Biopharma to study the cooperative anti-tumor effects deriving from the administered combined therapy of sintilimab and an innovative ADC candidate is gratifying. Such collaboration reinforces sintilimab's eminent role and the clinical significance it holds as a fundamental component in immunotherapy. Anticipating the combined modalities of these new drugs, our goal is to pioneer additional therapeutic pathways for individuals affected by cancer in the years ahead."
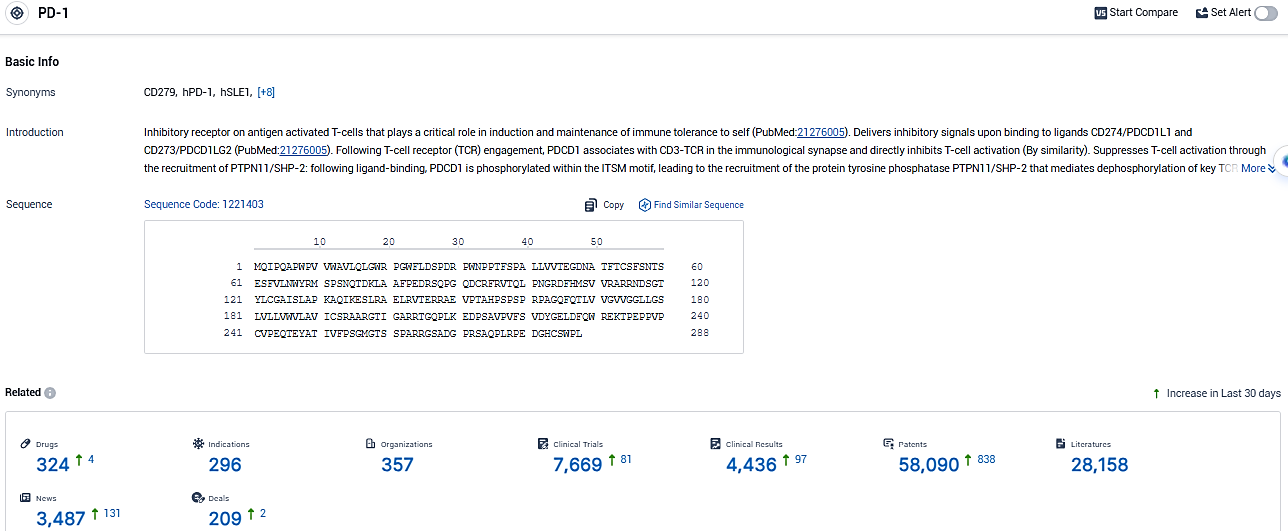
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 04, 2024, there are 324 investigational drugs for the PD-1 target, including 296 indications, 357 R&D institutions involved, with related clinical trials reaching 7669, and as many as 58090 patents.
Sintilimab has received approvals in China and globally for the treatment of various cancers and other diseases. The drug's targeting of PD-1 and its multiple regulatory designations highlight its potential as a breakthrough therapy in the field of biomedicine.