IO-108: A Quick Look at Its R&D Progress and Clinical Results from the 2023 AACR
On 14 Apr 2023, the first-in-human phase 1 trial of IO-108 as monotherapy and in combination with pembrolizumab in adult patients with advanced relapsed or refractory solid tumors was reported at the AACR Congress.
IO-108's R&D Progress
IO-108 is a monoclonal antibody drug developed by Immune-Onc Therapeutics, Inc. It specifically targets LILRB2 and is primarily intended for the treatment of neoplasms, particularly solid tumors.
According to the Patsnap Synapse, as of now, IO-108 has reached Phase 1 of clinical trials, both globally and in China. And the clinical trial areas for IO-108 are primarily in the United States and China. The key indication is Metastatic Solid Tumor. 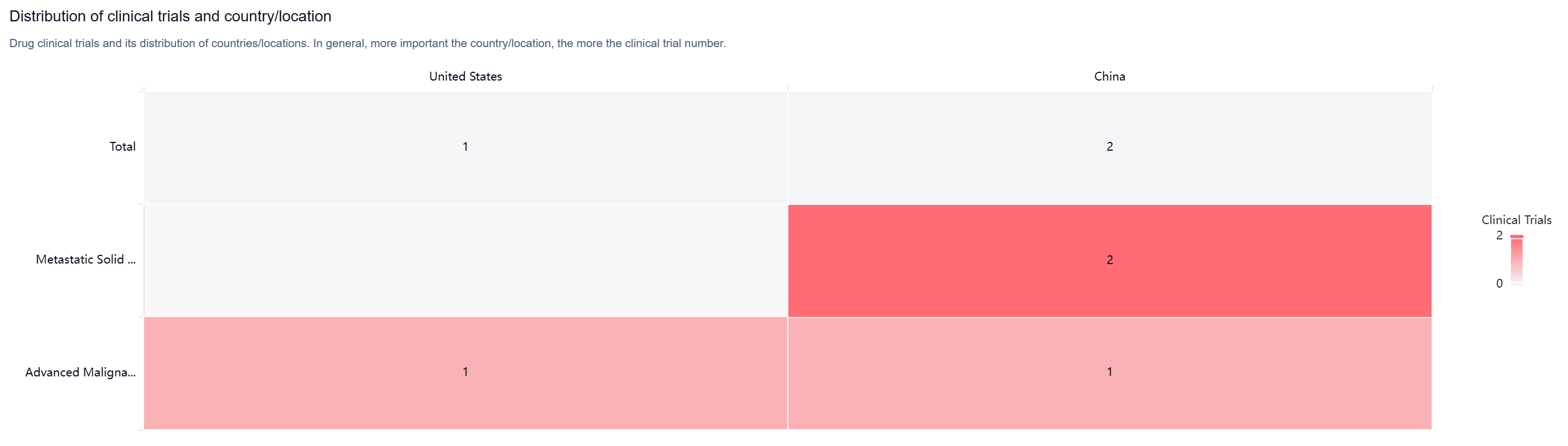
Detailed Clinical Result of IO-108
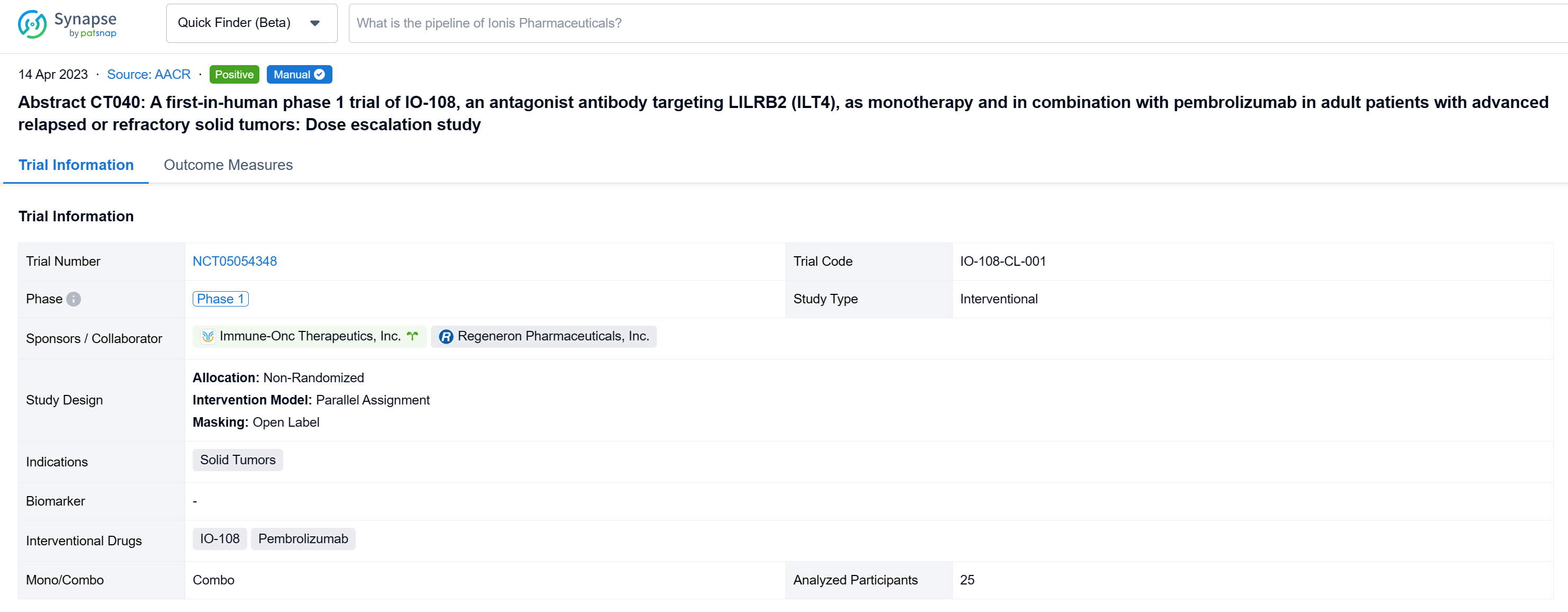
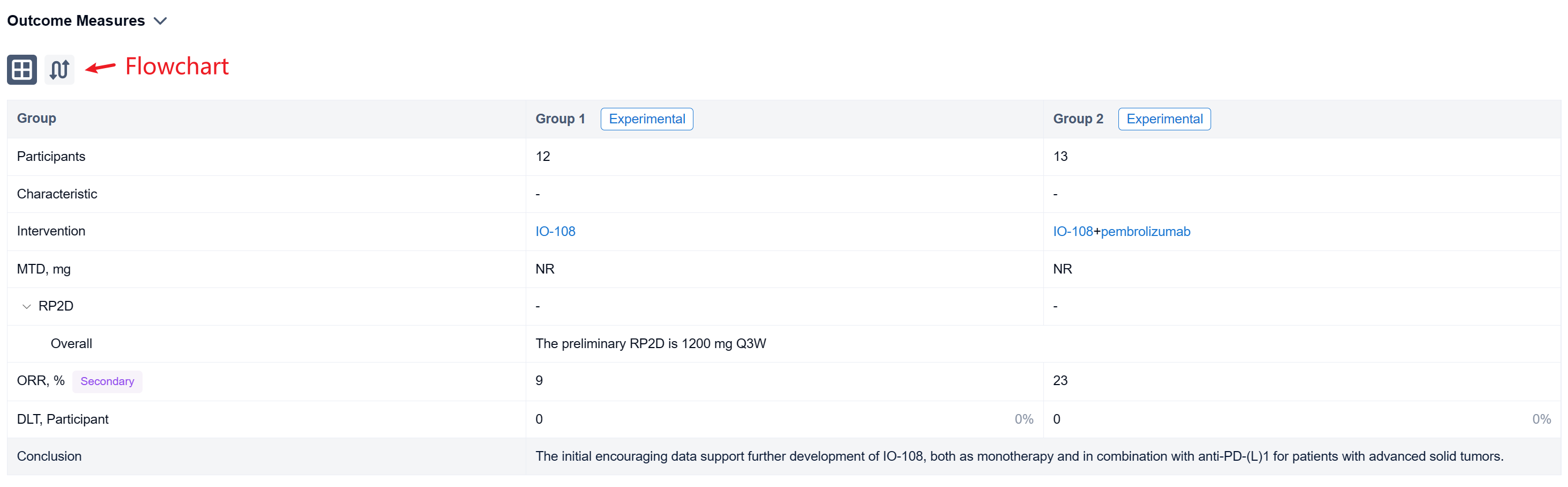
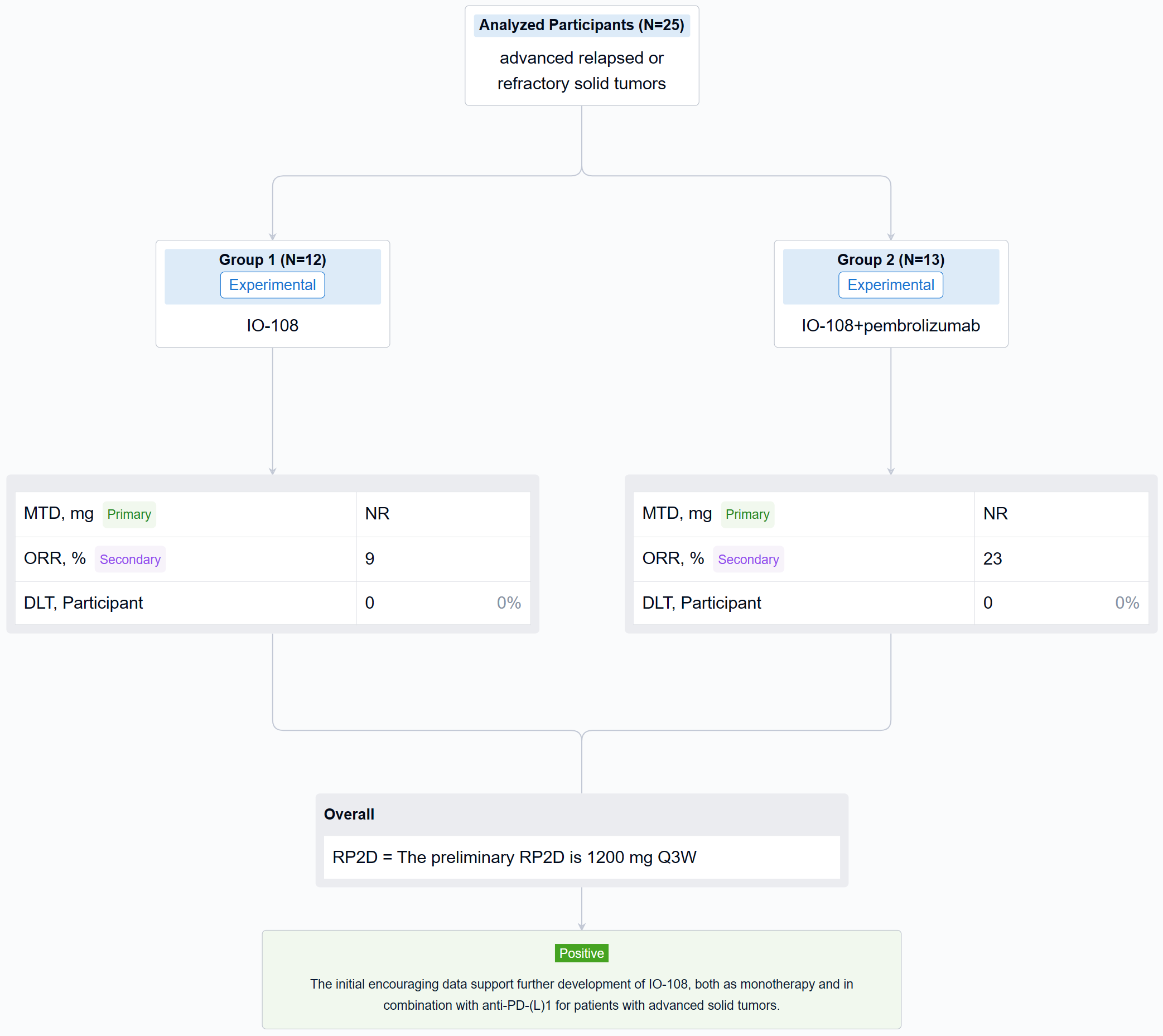
The non-randomized, parallel assignment, open-labeled clinical trial (NCT05054348) was a dose escalation study.
In this study, the dose escalation phase enrolled patients into escalating dose cohorts of IO-108 (60-1800 mg, Q3W IV) as monotherapy or in combination with pembrolizumab. Patients with disease progression on monotherapy could crossover to the combination arm. Primary objectives were safety and tolerability, and identification of the recommended phase 2 dose (RP2D). Secondary and exploratory objectives include evaluation of pharmacokinetics (PK), immunogenicity, pharmacodynamic (PD) biomarker effects and anti-tumor activity as measured by objective response rate (RECIST 1.1).
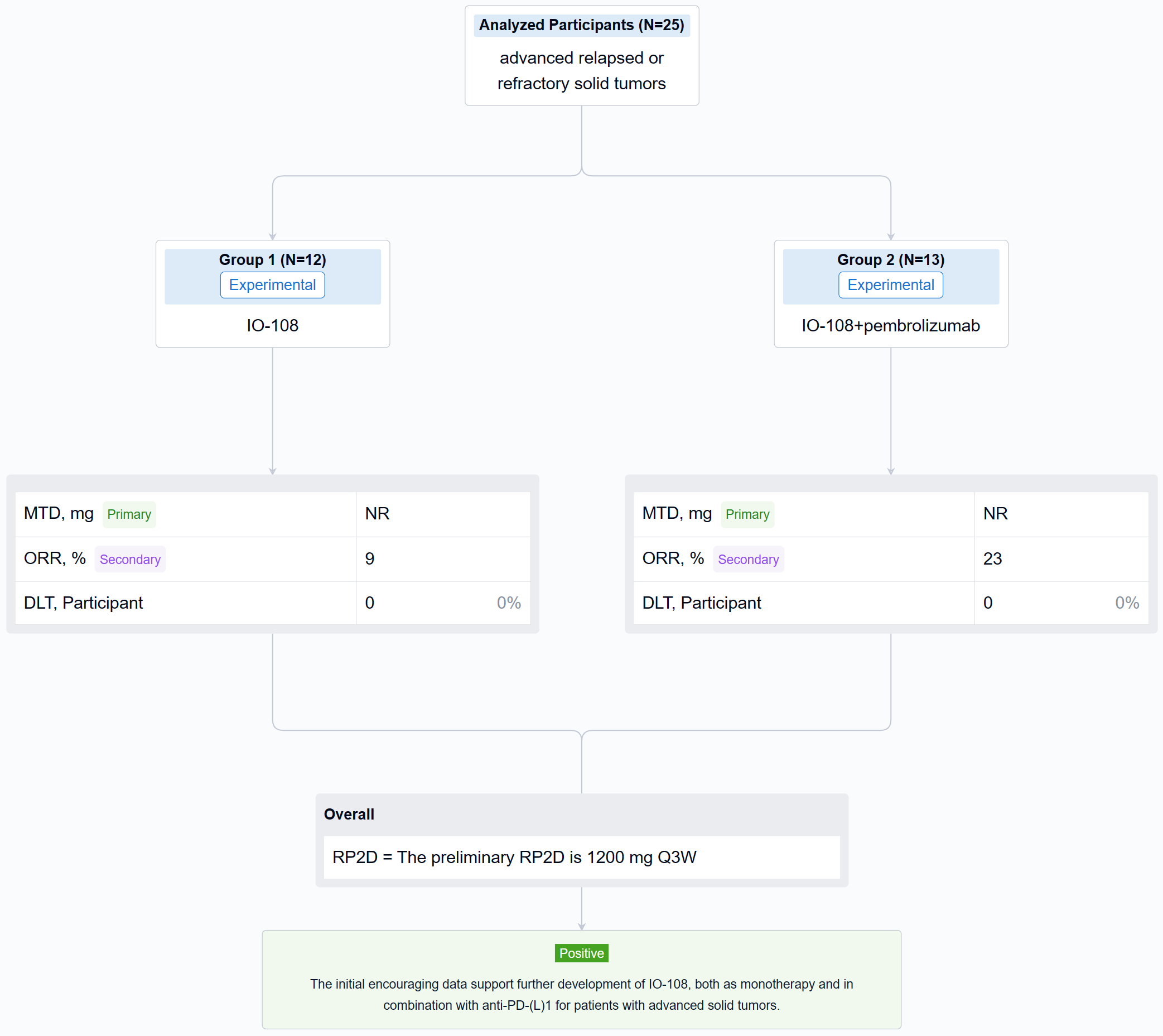
The result showed that twenty-five relapsed/refractory solid tumor patients (median 4.5 prior lines of therapy for monotherapy and 3.6 for combination therapy) were treated with IO-108 monotherapy (n=12) or IO-108 + pembrolizumab (n=13). IO-108 was well-tolerated up to the maximal administered dose of 1800 mg Q3W as monotherapy and in combination with pembrolizumab, with no SAEs related to IO-108 and no DLTs observed. Maximum tolerated dose (MTD) was not reached. Full receptor occupancy through 21 days was achieved at ≥600 mg. The preliminary RP2D is 1200 mg Q3W. Dose-expansion cohorts of IO-108 monotherapy and IO-108 + anti-PD-1 are ongoing. Twenty-three patients were efficacy evaluable (11 monotherapy, 12 combination therapy plus 1 crossover). Overall response rate was 9% in monotherapy cohort (1 Merkel cell carcinoma, prior pembrolizumab followed by nivolumab/ipilimumab) and 23% in combination therapy (2 cholangiocarcinoma, 1 MSS CRC with neuroendocrine features). The best overall response was 1 CR, 4 SD among monotherapy, and was 3 PR, 4 SD among combination therapy. The 4 responding patients remain on study with an on-going treatment duration of 8 to 12 months as of abstract submission. Consistent with the MOA, clinical benefit correlated with baseline characteristics and post-treatment changes in PD biomarkers including reprogramming of myeloid cells and activation of T cells.
It can be concluded that the initial encouraging data support further development of IO-108, both as monotherapy and in combination with anti-PD-(L)1 for patients with advanced solid tumors.
How to Easily View the Clinical Results Using Synapse Database?
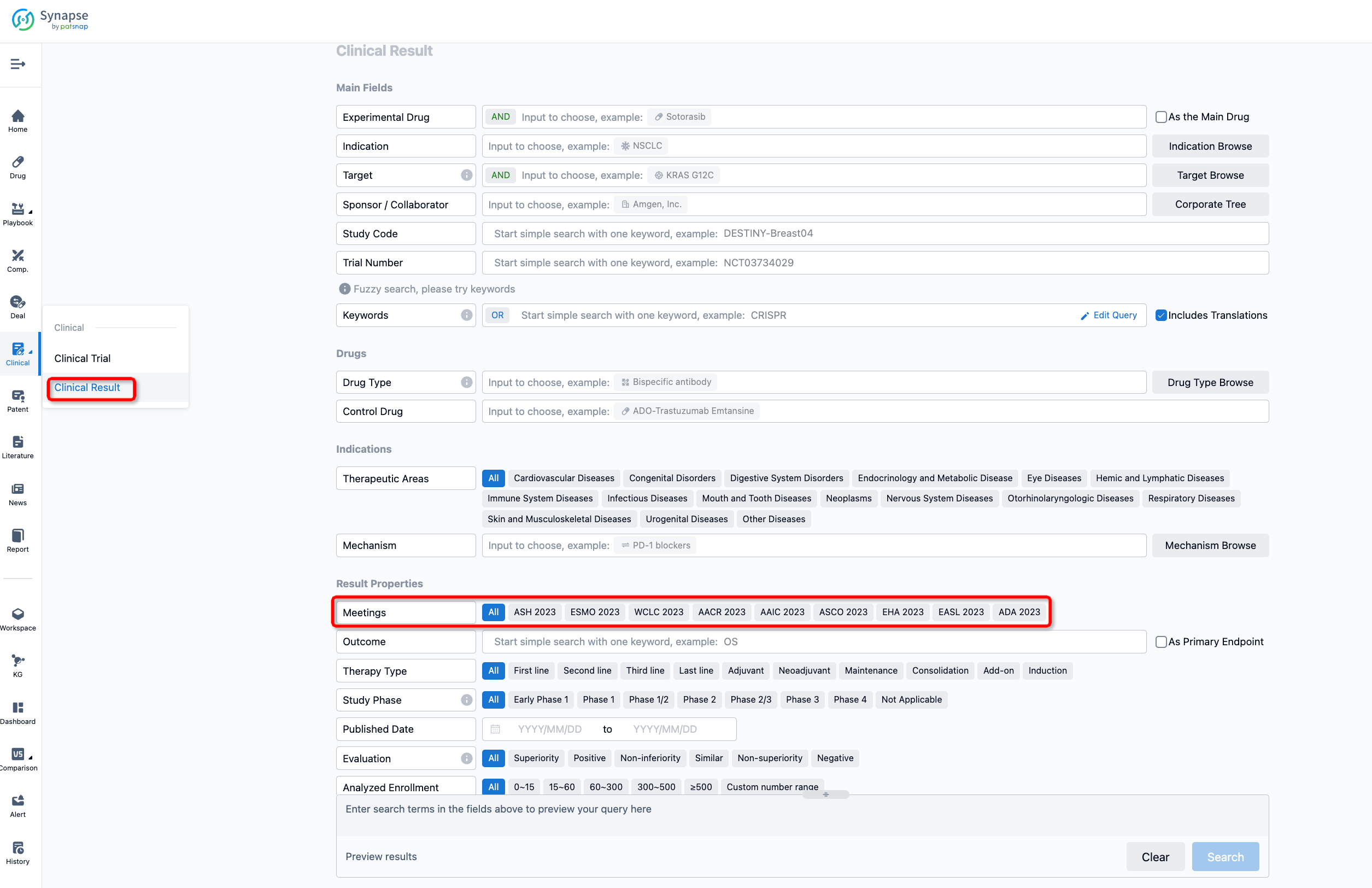
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
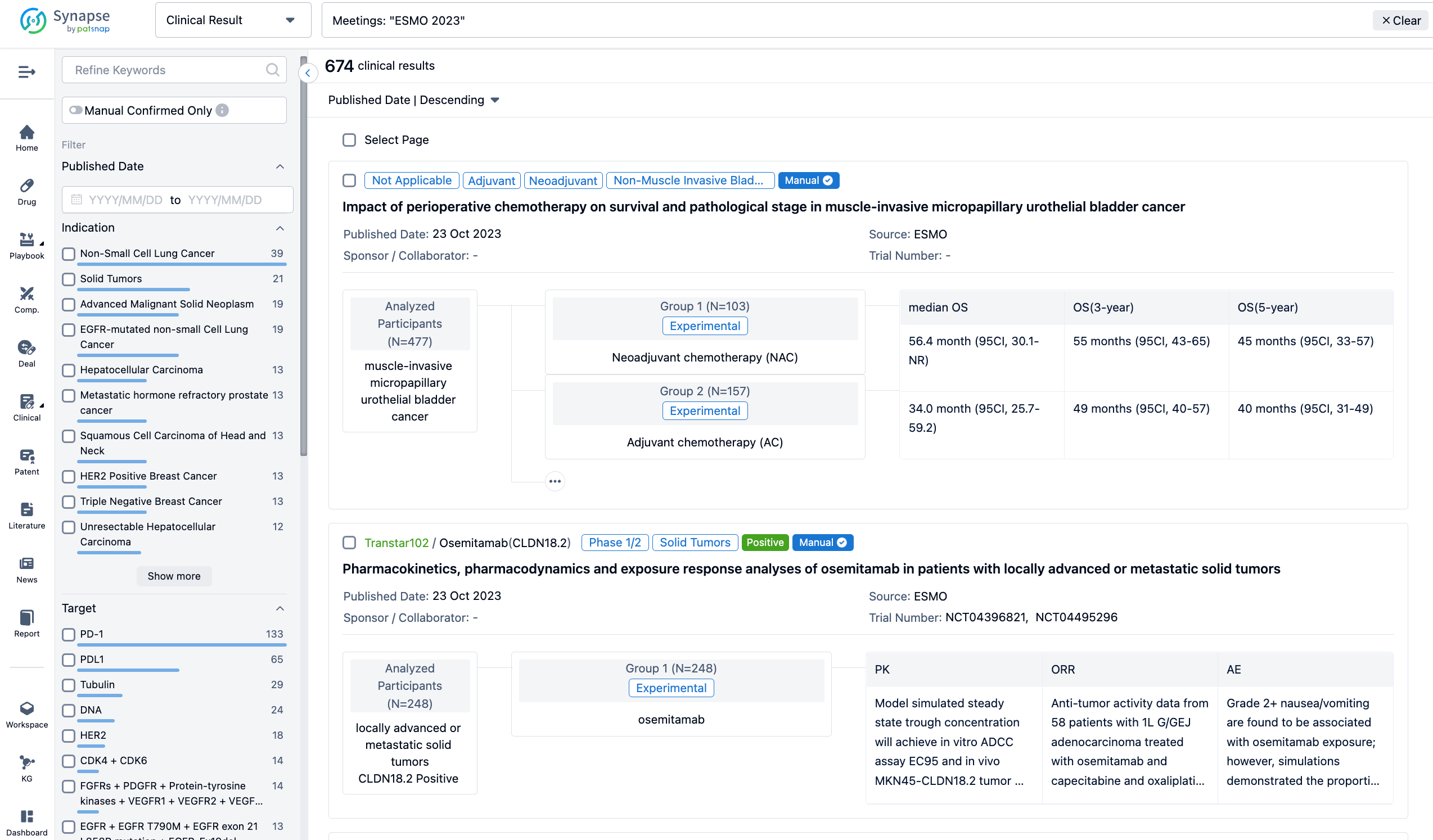
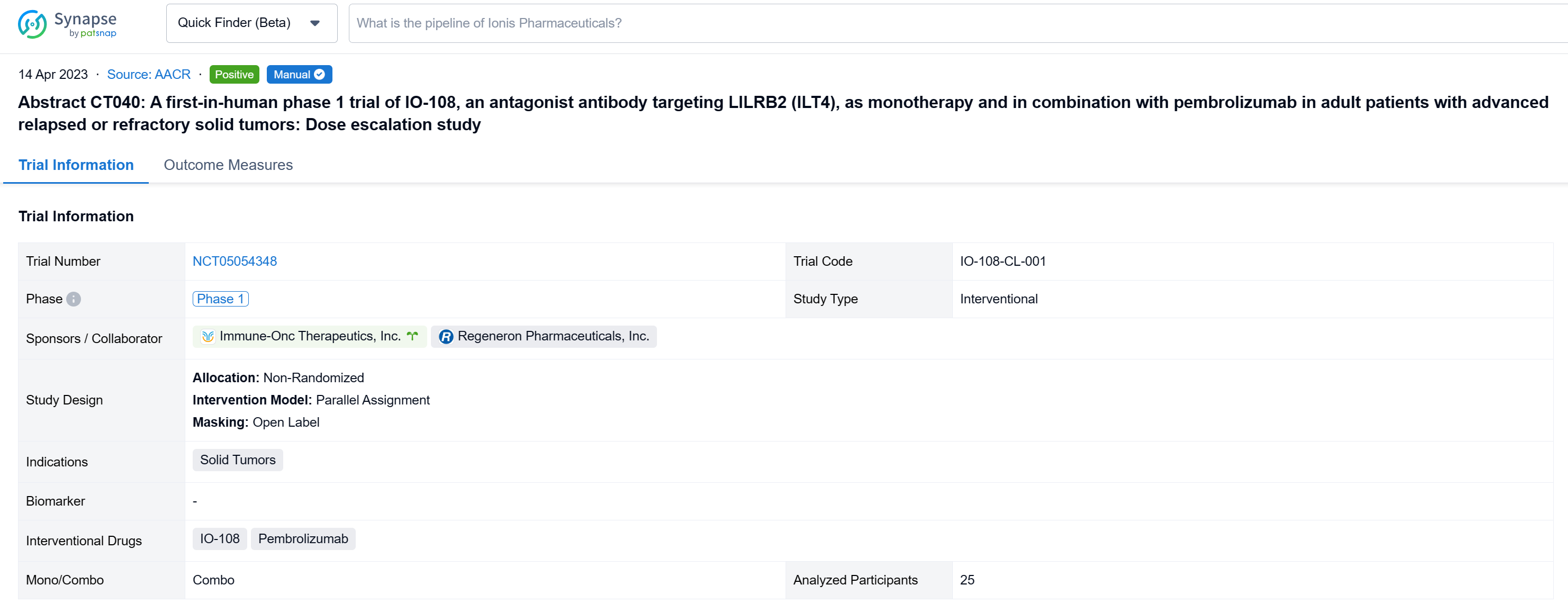
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
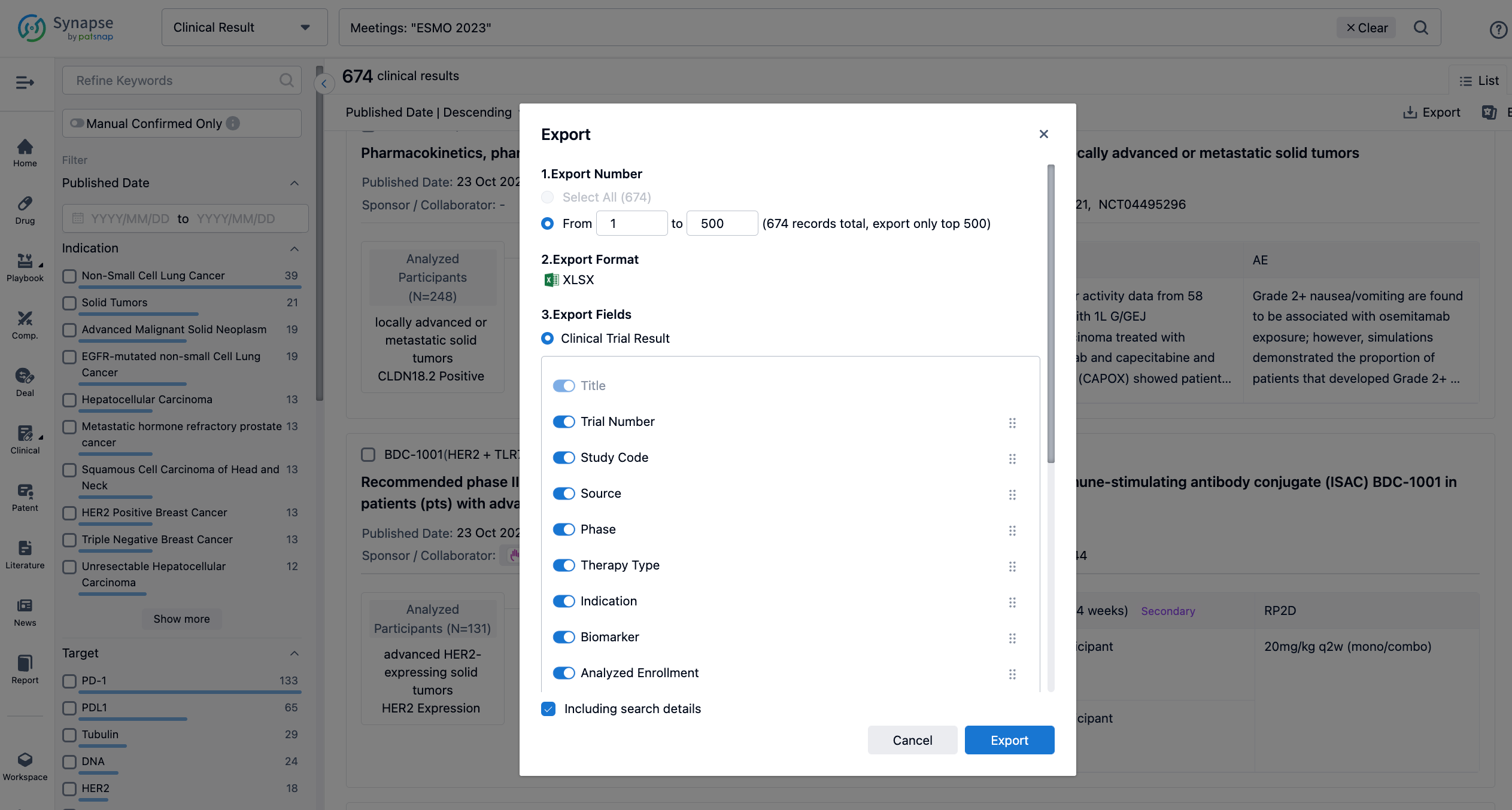
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!