Ironwood Pharma's Phase 2 STARGAZE Study Shows Promise for Apraglutide in Treatment-Resistant GI aGVHD
Ironwood Pharmaceuticals, Inc., a company specializing in gastrointestinal health, revealed encouraging initial outcomes for the initial 91 days in their Phase II STARGAZE study. The research is assessing the efficacy of apraglutide in individuals dealing with acute Gastrointestinal Graft-versus-Host Disease that does not respond to steroids.
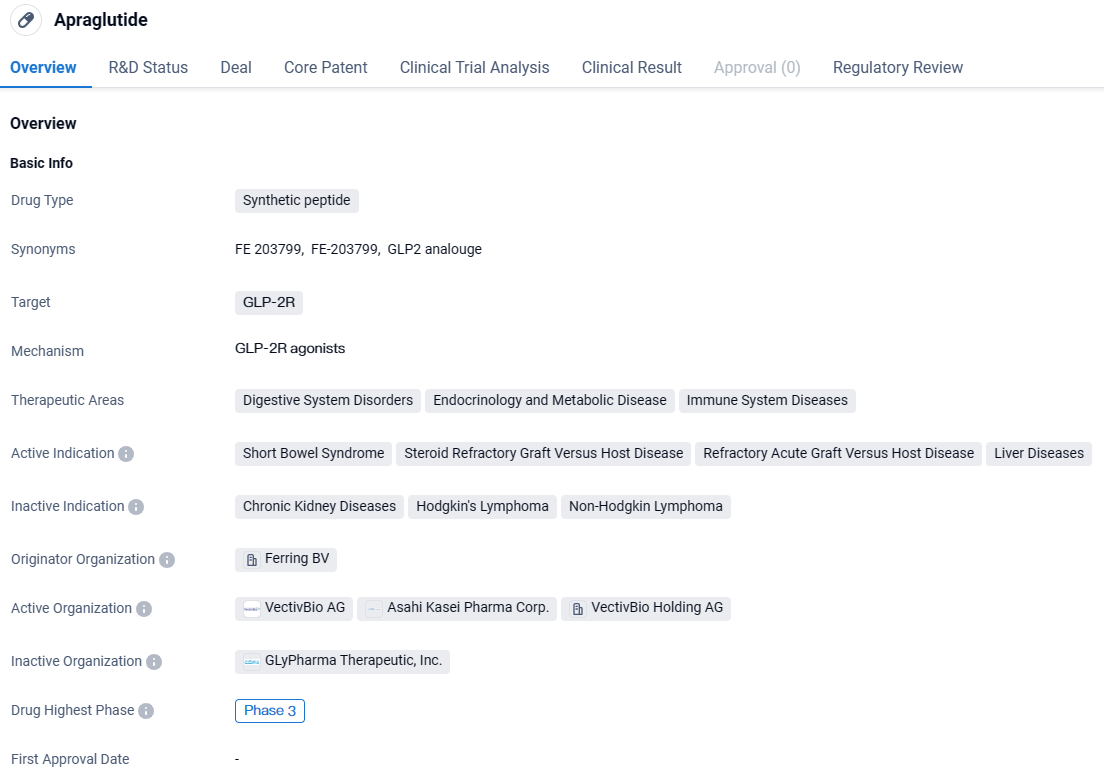
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The main aim of this clinical investigation was to assess the safety profile and the ability for patients to tolerate a weekly administration of apraglutide, a sophisticated, extended-release GLP-2 analog, in patients with steroid-refractory gastrointestinal acute GVHD who were receiving conventional treatments including systemic corticosteroids and ruxolitinib. This innovative research marked the inaugural integration of a GLP-2 analog with anti-rejection medication in managing SR GI aGVHD.
The security and patient acceptance recorded during this investigation aligned with expectations based on routine clinical procedures and the established safety benchmarks of GLP-2 analogs, along with the incidences recorded in patients with GVHD. The safety landscape for apraglutide within the context of SR GI aGVHD treatment was under continual surveillance by a separate safety oversight body, which gave their recommendation to proceed with the research unaltered.
Beyond the focus on safety, the study also investigated effectiveness through secondary outcome measures, looking at the response of the lower gastrointestinal tract and overall organ improvement since the start of the trial as defined by the grading scale from the Mount Sinai Acute GVHD International Consortium. The significant portion of the cohort showed a positive reaction to the therapeutic intervention by the 28th and 56th day. All individuals who saw an improvement in their lower GI condition on the 28th day sustained this progress until the 56th and 91st days, in light of available assessments for organ staging.
"The results we're sharing signify a significant development for individuals battling SR GI aGVHD, particularly when considering the restricted response duration provided by agents that are currently authorized," commented Robert Zeiser, M.D., a specialist in Hematology and Oncology at the University of Freiburg and Lead Researcher for the STARGAZE study. “Considering apraglutide's potential to promote tissue regeneration and enhance gastrointestinal functionality, it may play a vital part, via a non-suppressive immunologic method, in the advanced treatment of acute GI GVHD. We're hopeful about what the long-term results will reveal for this group of patients.”
Further insights from the STARGAZE trial will be unveiled at an imminent healthcare conference. The STARGAZE research will advance to its two-year mark, enabling a subsequent analysis of safety and therapeutic effectiveness of apraglutide.
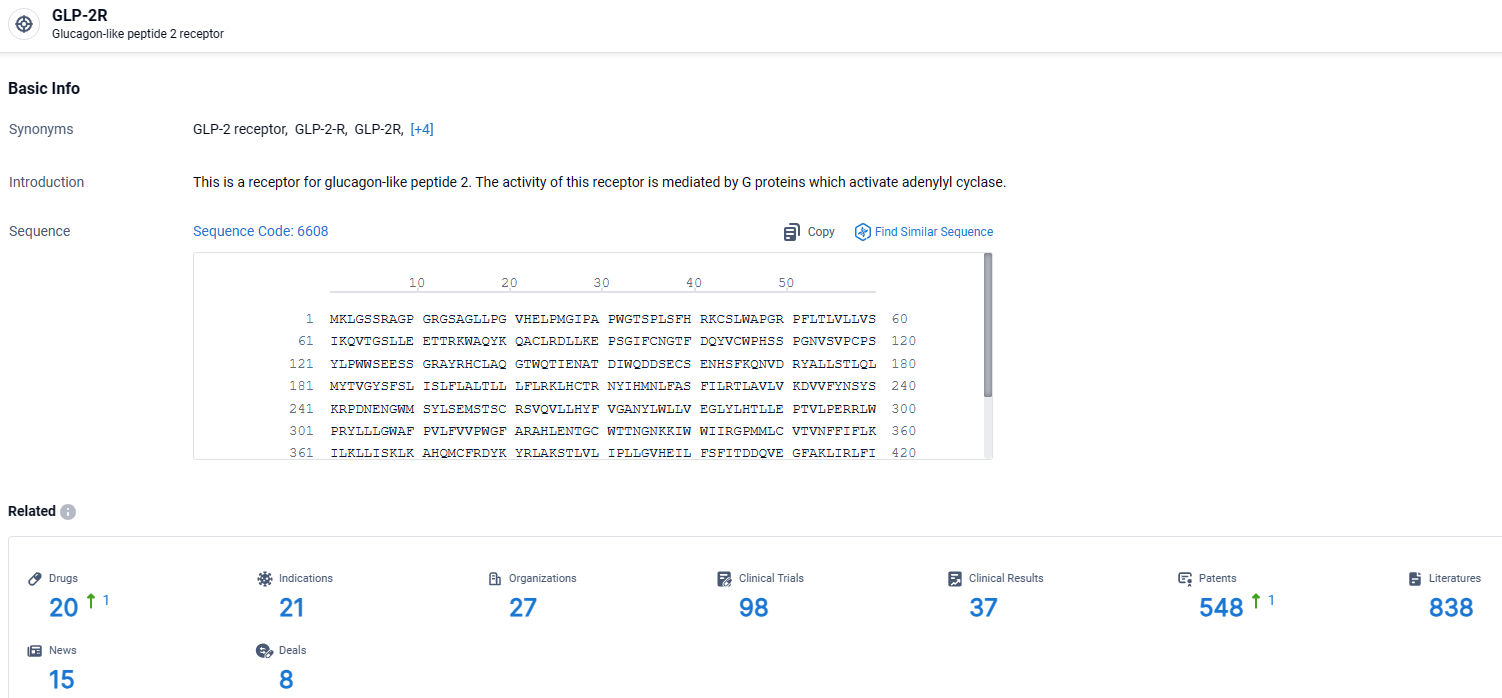
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 1, 2024, there are 20 investigational drugs for the GLP-2R target, including 21 indications, 27 R&D institutions involved, with related clinical trials reaching 98, and as many as 548 patents.
Apraglutide is a synthetic peptide drug that targets the GLP-2R receptor and is being developed for the treatment of various disorders in the fields of digestive system disorders, endocrinology and metabolic disease, and immune system diseases. It has shown promise in treating conditions such as short bowel syndrome, steroid refractory graft versus host disease, refractory acute graft versus host disease, and liver diseases.