January 2025: The Emergence of China’s Innovative Pharmaceutical Industry on the Global Stage
In January 2025, China’s innovative pharmaceutical sector continues to make its mark on the global stage. The collaboration between Innovent Biologics and Roche marks the international launch of IBI3009, an antibody-drug conjugate (ADC) targeting small cell lung cancer (SCLC). Meanwhile, the partnership between Duality Biologics and Avenzo Therapeutics concerning the EGFR/HER3 bispecific ADC, DB-1418/AVZO-1418, further demonstrates China’s progress in developing complex and efficient cancer therapies. As Lepu Biopharma and ArriVent BioPharma enter into a global exclusive licensing agreement for the ADC MRG007 targeting gastrointestinal cancers, Chinese pharmaceutical companies are transitioning from mere producers to key providers of global healthcare solutions.
Entering 2025, with the signing of several major collaboration agreements and the backing of relevant policies, Chinese pharmaceutical companies are at a new starting point. They are no longer confined to the domestic market but are actively seeking international collaborations to promote their innovative products and services worldwide.
1.Innovent Biologics Partners with Roche to Propel IBI3009 as a Revolutionary Treatment for Small Cell Lung Cancer
On January 2, 2025, Innovent Biologics (01801.HK) announced the global exclusive licensing of IBI3009 to Roche. Under the agreement, Innovent grants Roche the exclusive global rights for the development, production, and commercialization of IBI3009. Both companies will jointly oversee the early development of this ADC (antibody-drug conjugate), while Roche will be responsible for subsequent clinical development. Innovent will receive an upfront payment of $80 million, with potential development and commercialization milestone payments of up to $1 billion, plus tiered royalties based on global annual sales.
IBI3009 is a next-generation ADC developed by Innovent Biologics, specifically targeting Delta-like ligand 3 (DLL3). DLL3 is minimally expressed in normal tissues but is significantly overexpressed in certain cancers, particularly SCLC and other neuroendocrine tumors, making it a promising therapeutic target. By specifically binding to DLL3, IBI3009 can accurately attack tumor cells without harming healthy cells, offering a new treatment option for patients with advanced small cell lung cancer.
DLL3 is a member of the Notch signaling pathway, which includes Delta and Serrate/LAG-2-like molecules involved in critical cell-to-cell communication. The Notch pathway plays a vital role in cellular fate determination, differentiation, and proliferation. In certain cancer types, especially SCLC, DLL3 is abnormally overexpressed and is closely linked to tumor progression. Notably, while DLL3 is scarcely expressed in normal tissues, its significant presence in SCLC and other neuroendocrine tumors provides an ideal target for therapy. Because of its specific expression pattern, therapies targeting DLL3 can precisely identify and destroy cancer cells while minimizing side effects on healthy tissues.
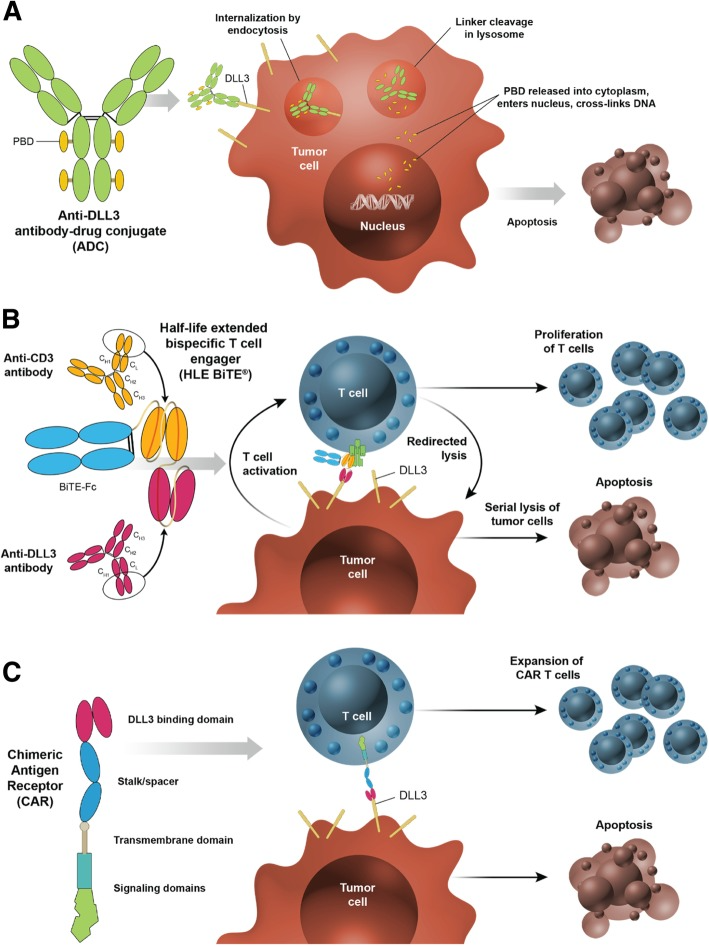
IBI3009 is designed as an innovative treatment based on the concept of ADCs, combining a potent small-molecule toxin with a monoclonal antibody that specifically recognizes DLL3. This complex enables precise targeting of DLL3-expressing tumor cells and is internalized by the cells through endocytosis. Once inside, the chemical bond connecting the antibody and the toxin breaks, releasing a novel topoisomerase I inhibitor (TOPO1i), which interferes with the essential function of topoisomerase I in DNA replication. This results in the accumulation of DNA damage and triggers apoptosis or direct cell death in tumor cells.
Additionally, in multiple mouse tumor-bearing models, particularly those resistant to chemotherapy, IBI3009 has shown promising antitumor activity and favorable safety characteristics. The drug has received IND approvals in Australia, China, and the United States, and in December 2024, the first patient was dosed in a Phase I clinical trial, marking an important step toward its market introduction.
While Amgen’s Tarlatamab, a CD3/DLL3 bispecific antibody, has already been approved for commercialization, IBI3009, with its unique ADC structure and potentially superior efficacy and safety, is expected to stand out in the competitive market. Currently, aside from Tarlatamab, other DLL3-targeting therapies are mostly in Phase II clinical trials or earlier, suggesting significant room for growth in this field. With the global exclusive collaboration and licensing agreement between Innovent and Roche, IBI3009 has gained support from an international pharmaceutical giant, providing a strong boost for its subsequent clinical development and commercialization.
2.DualityBio and Avenzo Therapeutics Enter into a Major Bipsecific ADC Collaboration Agreement
On January 8, 2025, DualityBio reached an exclusive licensing agreement with Avenzo Therapeutics to grant Avenzo the global rights (excluding Greater China) to develop, manufacture, and commercialize DualityBio's EGFR/HER3 bispecific antibody-drug conjugate (ADC), DB-1418/AVZO-1418. Under the agreement, DualityBio will receive an upfront payment of $50 million, and is eligible to receive up to $1.15 billion in development, regulatory, and commercial milestones. Additionally, DualityBio will receive royalties on sales within Avenzo’s territories.
DualityBio's DB-1418/AVZO-1418 is an innovative bispecific antibody-drug conjugate that simultaneously targets two key tumor-associated antigens - EGFR and HER3. EGFR (Epidermal Growth Factor Receptor) and HER3 (Human Epidermal growth factor Receptor 3) are overexpressed in various cancer types. EGFR, a member of the ErbB receptor family, plays a crucial role in regulating cell proliferation, differentiation, and survival. Mutations or overexpression of EGFR can promote growth and spread of cancer cells, especially in non-small cell lung cancer and head and neck cancers. HER3, although lacking kinase activity, can form heterodimers with other ErbB family members, activating downstream signaling pathways including the PI3K/Akt pathway, which is vital for cancer cell survival. HER3 is also often overexpressed in certain types of breast cancer and other solid tumors, where high expression is associated with poor prognosis.
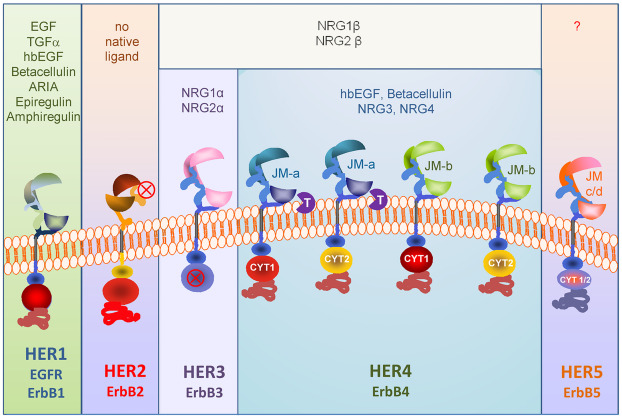
DB-1418 is uniquely designed to target both EGFR and HER3, capitalizing on their co-expression on the same tumor cells. By combining recognition of EGFR and HER3, DB-1418 not only enhances selective targeting of cancer cells but may also overcome resistance issues associated with single-target therapies. This bispecific strategy helps to more comprehensively block ErbB family-mediated signaling, thus offering potentially more effective anticancer effects.
DB-1418 is constructed using DualityBio’s proprietary DIBAC platform, ensuring that the antibody component effectively binds the targets and stably carries the cytotoxic drug. This ADC consists of an antibody specific for EGFR/HER3 linked to a potent cytotoxic small molecule – a topoisomerase I inhibitor. Once DB-1418 binds to EGFR and HER3 on the surface of tumor cells, it is internalized into the cell. During this process, DB-1418 enhances internalization, enabling direct delivery of the cytotoxic payload into the tumor cells.
Inside the cell, due to specific acidic conditions or enzymatic action, the linker releases the topoisomerase I inhibitor. This inhibitor disrupts a critical step in the DNA replication process, causing accumulation of DNA damage and ultimately triggering apoptosis in cancer cells. Furthermore, the design of DB-1418 addresses conventional resistance issues seen in single-target therapies by utilizing a dual targeting mechanism, enhancing the likelihood of treatment success and duration. Currently, DB-1418 is undergoing IND-enabling studies and is planned to enter clinical trials within the year 2025, offering new hope for treatment to patients.
3.Lepu Biopharma collaborates with ArriVent to advance the globalization of the MRG007 antibody-drug conjugate
On January 22, 2025, Lepu Biopharma announced a significant collaboration with ArriVent BioPharma, entering into an exclusive global licensing agreement for the innovative antibody-drug conjugate (ADC) MRG007, targeting gastrointestinal cancers. Under this agreement, ArriVent has been granted the rights to develop, manufacture, and commercialize MRG007 outside of the Greater China area, including Mainland China, Hong Kong, Macau, and Taiwan. This partnership marks a critical step in Lepu Biopharma's global strategy and is expected to accelerate the research and development process of MRG007 with the expertise and resources of ArriVent.
MRG007 is a potential best-in-class ADC designed to target specific antigens in gastrointestinal cancers. While the specific target has not yet been disclosed, the drug is known to effectively bind and deliver cytotoxic agents into tumor cells, thus achieving precise targeting of cancer cells. Preclinical studies have demonstrated that MRG007 exhibits potent anti-tumor activity in models of gastrointestinal cancers and has a high therapeutic index, meaning it can provide effective treatment while minimizing damage to normal tissues. The first Investigational New Drug (IND) application is planned for submission in the first half of 2025, with initial clinical development focusing on colorectal cancer, pancreatic cancer, and other gastrointestinal malignancies, which are often challenging to treat. Thus, the progress of MRG007 represents significant hope for patients.
From a commercial perspective, this partnership has brought substantial economic benefits and development opportunities to Lepu Biopharma. According to the terms of the agreement, Lepu Biopharma will receive an upfront payment and near-term milestone payments totaling $47 million, along with up to $1.16 billion in potential development, regulatory, and sales milestone payments. Additionally, Lepu Biopharma will receive tiered royalties on net sales from regions outside Greater China. This structure not only ensures an immediate influx of funds for Lepu Biopharma but also lays the groundwork for future success. Through this collaboration, Lepu Biopharma and ArriVent are jointly committed to advancing the research and development of MRG007, striving to bring new treatment options to patients with gastrointestinal cancers worldwide.
Summary
As we enter 2025, the path to internationalization for China's pharmaceutical industry appears more defined and hopeful. With a series of significant partnership agreements and supportive policies in place, Chinese pharmaceutical companies stand at a new starting point. The collaboration between Innovent Biologics and Roche for the ADC IBI3009 targeting small cell lung cancer marks its official international debut. Meanwhile, agreements like the one between Yingene Biopharma and Avenzo Therapeutics for the EGFR/HER3 bispecific ADC DB-1418/AVZO-1418 further demonstrate China's advancements in developing complex and effective anti-cancer therapies. With the exclusive global licensing agreement reached between Lepu Biopharma and ArriVent BioPharma for the ADC MRG007 for treating gastrointestinal cancers, Chinese pharmaceutical entities are increasingly transitioning from mere producers to significant global healthcare solution providers. China's pharmaceutical industry is no longer confined to the domestic market but actively seeks international collaborations, a historic opportunity both for elevating global patient care and for driving the upgrade of China's own pharmaceutical industry.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
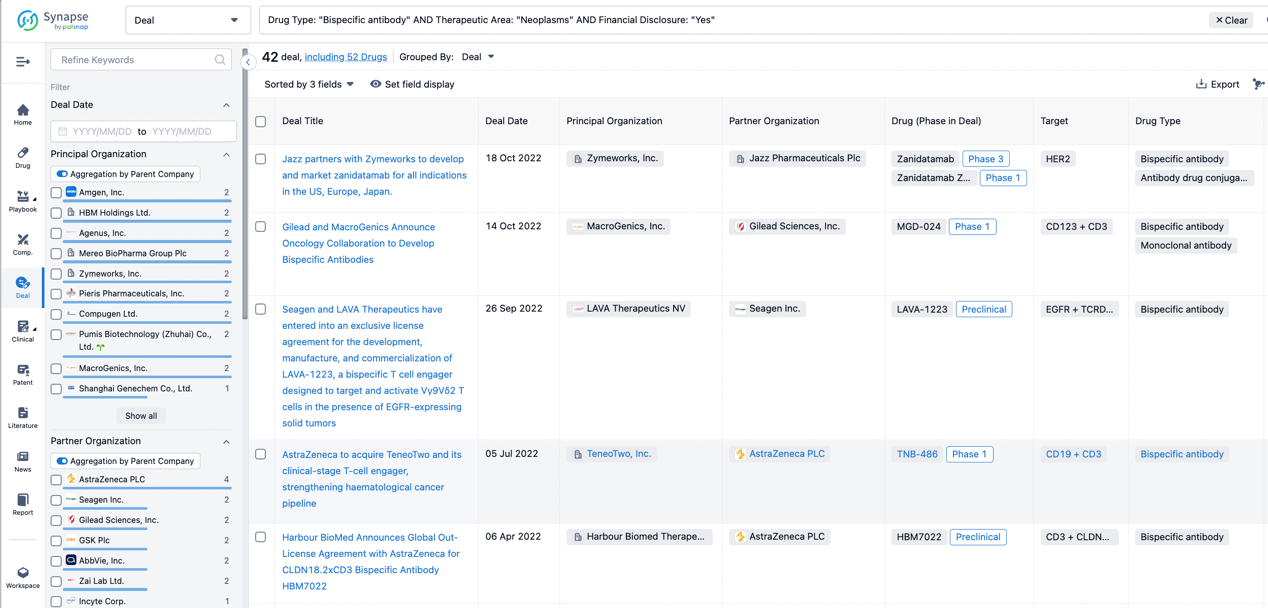
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
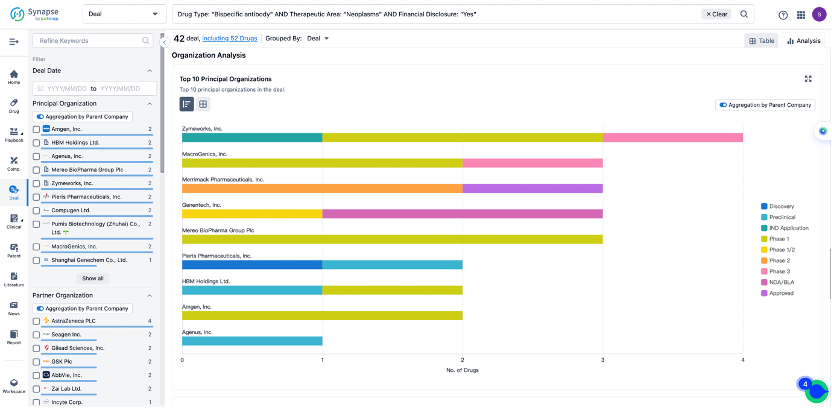
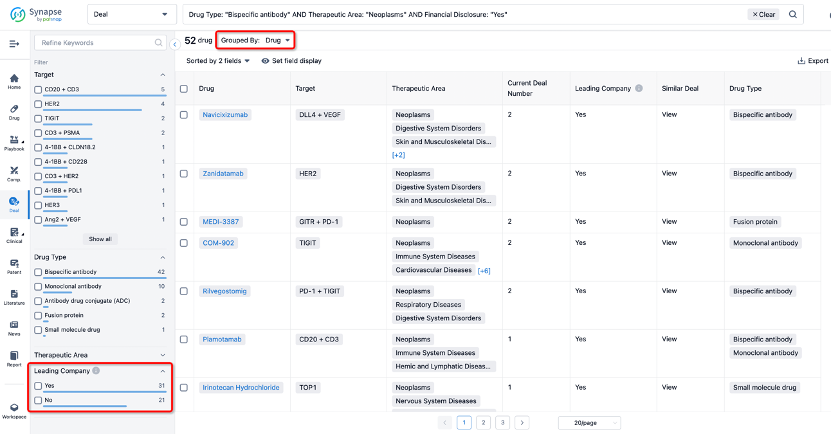
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
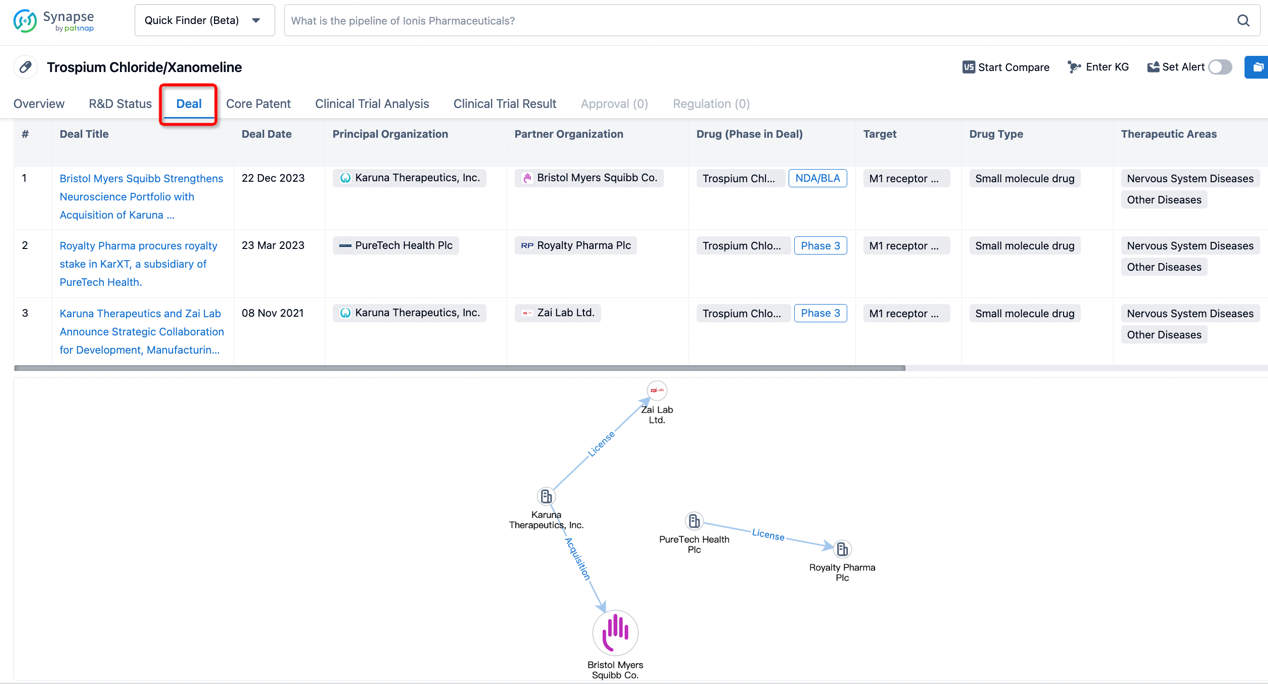
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to explore new pharmaceutical funding transactions!
Reference
- 1. Owen DH, Giffin MJ, Bailis JM, Smit MD, Carbone DP, He K. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019 Jun 18;12(1):61. doi: 10.1186/s13045-019-0745-2. PMID: 31215500; PMCID: PMC6582566.
- 2. Brockhoff G. "Shedding" light on HER4 signaling in normal and malignant breast tissues. Cell Signal. 2022 Sep;97:110401. doi: 10.1016/j.cellsig.2022.110401. Epub 2022 Jul 9. PMID: 35820544.