January 2025 Wave of Mergers and Acquisitions in the Biotechnology and Pharmaceutical Industry
In January 2025, several significant mergers and acquisitions (M&A) took place in the biopharmaceutical industry. These transactions not only reflect the strategic positioning of major companies in specific therapeutic areas but also demonstrate the industry's continued focus on innovative technologies and unmet medical needs. Lantheus Holdings, a leader in the field of radiopharmaceuticals, has significantly strengthened its market position and technological portfolio through a series of acquisitions. First, Lantheus completed the acquisition of Evergreen Theragnostics, bringing advanced manufacturing infrastructure and the PET drug Octevy, used for diagnosing neuroendocrine tumors, thereby further enriching its product line. Subsequently, Lantheus plans to acquire Life Molecular Imaging (LMI) for $350 million in an upfront payment, aiming to enhance its competitiveness in the Alzheimer's disease diagnostic market and expand its influence in oncology.
At the same time, other major pharmaceutical companies are taking action to consolidate their leadership in key areas. Johnson & Johnson, Eli Lilly, and GlaxoSmithKline (GSK) have each strengthened their product portfolios in neuroscience, precision oncology, and gastrointestinal cancer treatment by acquiring Intra-Cellular Therapies, Scorpion Therapeutics, and IDRx, respectively. Additionally, the acquisition of Tavros Therapeutics by Vividion Therapeutics and the merger between Salarius Pharmaceuticals and Decoy Therapeutics reflect the industry's emphasis on cutting-edge technological platforms, particularly those offering differentiated advantages.
Notably, the sale of Serina Therapeutics' subsidiary UniverXome Bioengineering to Juvenescence illustrates that, when facing financial challenges, asset restructuring and strategic partnerships can be essential strategies for companies. Although Biogen's non-binding acquisition proposal for Sage Therapeutics was ultimately rejected, it highlighted the importance of the existing collaboration between the two companies and the potential for deeper integration.
1.Lantheus Announces Completion of Acquisition of Evergreen Theragnostics
On January 29, 2025, U.S. radiopharmaceutical company Lantheus announced the completion of its acquisition of Evergreen Theragnostics for a total transaction value of $1 billion, which includes an upfront payment of $250 million and potential additional installment payments of up to $752.5 million. This marks Lantheus' fifth acquisition since January 2024, aimed at enhancing its production capacity and technological capabilities in the radiopharmaceuticals sector. The acquisition enables Lantheus to gain Evergreen's radioligand therapy (RLT) manufacturing infrastructure, which supports both clinical trial needs and large-scale commercial production, reducing dependence on third-party manufacturing partners.
The acquisition also includes Octevy, a PET diagnostic drug currently in the registration phase, used for detecting neuroendocrine tumors in both adult and pediatric patients. Lantheus noted that Octevy could serve as a complement to PNT2003 (lutetium Lu 177 edotreotide), a therapeutic drug developed by the company in collaboration with POINT Biopharma, which is currently undergoing Phase III trials for neuroendocrine tumors. This combination provides Lantheus with a more comprehensive product line in the neuroendocrine tumor treatment field, enhancing the company's competitiveness in this area.
2.Lantheus Holdings Announces Plan to Acquire Life Molecular Imaging (LMI) for $350 Million Upfront
On January 17, 2025, Lantheus Holdings announced its plan to acquire Life Molecular Imaging (LMI) for an upfront payment of $350 million, with the potential for additional milestone and contingent payments of up to $400 million. This strategic acquisition aims to strengthen Lantheus' position in the Alzheimer's disease diagnostic market, particularly by acquiring LMI's key product, Neuraceq, a commercial diagnostic agent used to detect β-amyloid plaques in Alzheimer's patients.
The transaction brings five significant assets to Lantheus, with Neuraceq, as a commercialized product, enabling the company to enter the Alzheimer's diagnostic market at least one year ahead of schedule. In addition, Lantheus will gain access to a diagnostic and therapeutic radiopharmaceutical combination targeting the gastrin-releasing peptide receptor, which is of particular relevance to breast and prostate cancers. These actions not only reinforce Lantheus' presence in the neurodegenerative disease diagnostic space but also complement the company's existing oncology product line, such as the prostate cancer diagnostic drug Pylarify.
Through this acquisition, Lantheus further solidifies its leadership position in the radiopharmaceutical industry and injects new vitality into its diagnostic capabilities in Alzheimer's and other disease areas. This move reflects Lantheus' commitment to advancing medical imaging technologies and its dedication to developing more accurate and effective diagnostic tools, which is expected to have a positive impact on early detection and improving patient outcomes.
3.Serina Therapeutics Announces Sale of Subsidiary UniverXome Bioengineering to Juvenescence
On January 16, 2025, Serina Therapeutics announced the sale of its subsidiary UniverXome Bioengineering to Juvenescence, a company focused on anti-aging, in order to repay a total debt of $11.2 million. This transaction is aimed at alleviating Serina's financial burden in the new year and refocusing its resources on its core business. UniverXome holds assets that were left over from the reverse merger with AgeX Therapeutics, a publicly traded company that has long faced financial difficulties.
Before merging with Serina, AgeX Therapeutics had repeatedly warned that its funds were running out and had been seeking solutions, ultimately completing a merger plan with Serina in August 2023. While this merger provided a lifeline for AgeX, it also transferred the associated financial pressure to Serina. Notably, Juvenescence had previously provided tens of millions of dollars in loans to AgeX, indicating its interest in and support for AgeX and its technologies.
This asset sale is not focused on specific drugs or therapeutic areas but revolves around addressing the complex financial relationships and asset restructuring between the companies. By selling UniverXome to Juvenescence, Serina not only reduces its debt burden but also allows Juvenescence to acquire certain valuable biotechnology technologies or intellectual property previously held by AgeX, although specific details were not disclosed in the announcement. This move reflects a broader trend in the biotechnology industry of addressing financial challenges through asset restructuring and strategic partnerships.
4.Johnson & Johnson Announces Acquisition of Intra-Cellular Therapies
On January 13, 2025, Johnson & Johnson announced the acquisition of Intra-Cellular Therapies to strengthen its leadership in the neuroscience field. The core of this acquisition is CAPLYTA® (lumateperone), the first and only FDA-approved drug for the adjunctive and monotherapy treatment of bipolar I and II depression, as well as for the treatment of schizophrenia in adults. This acquisition not only enhances Johnson & Johnson's product line in neuroscience but also lays the foundation for sales growth over the next few years.
As an important treatment option, CAPLYTA® has already been submitted for a supplemental new drug application (sNDA) to expand its use as an adjunctive treatment for major depressive disorder (MDD). If approved, CAPLYTA® is expected to become one of the standard treatments for most common types of depression, further expanding its market influence. Moreover, CAPLYTA®'s peak annual sales potential exceeds $5 billion, significantly boosting Johnson & Johnson's financial performance and helping the company exceed analysts' growth expectations.
In addition to its current indications, CAPLYTA® has shown potential in clinical studies for generalized anxiety disorder, Alzheimer's-related psychosis, and agitation symptoms. These additional indications not only offer a broader market prospect for CAPLYTA® but also demonstrate Johnson & Johnson's commitment to developing treatments for a variety of neurological disorders. Through this strategic move, Johnson & Johnson is steadily reinforcing its leadership in the neuroscience sector.
5.Eli Lilly Announces Final Acquisition Agreement with Scorpion Therapeutics
On January 13, 2025, Eli Lilly announced that it had reached a final agreement to acquire Scorpion Therapeutics and its development of the mutation-selective PI3Kα inhibitor project, STX-478. STX-478 is a once-daily oral medication currently in Phase 1/2 clinical trials, aimed at treating 30-40% of hormone-positive breast cancer patients, with the potential to become a best-in-class treatment option. This acquisition will enhance Eli Lilly's oncology portfolio and provide new momentum for its development in precision oncology.
Under the terms of the agreement, Eli Lilly will pay up to $2.5 billion in cash to complete the acquisition of Scorpion, including upfront payments and subsequent payments based on achieving specific regulatory and sales milestones. Additionally, Scorpion will spin off a new entity that will hold assets related to its employees and non-PI3Kα projects, which will be owned by existing shareholders and led by Scorpion's current management team, focusing on the discovery and delivery of precision drug combinations. Through this transaction, Eli Lilly aims to leverage Scorpion's expertise and its own to accelerate the development of STX-478, improving treatment outcomes for patients with various solid tumors driven by PI3Kα mutations.
STX-478, as a differentiated mutation-selective PI3Kα inhibitor, selectively targets the PI3Kα pathway in cancer cells rather than healthy cells, overcoming key limitations of existing drugs targeting this pathway. This characteristic makes STX-478 poised to provide deeper pathway inhibition and higher tolerance, offering hope for patients in need of effective and safe targeted therapies for the PI3Kα pathway. Eli Lilly hopes to make significant progress in the treatment of breast cancer and other advanced solid tumors through this acquisition.
6.GlaxoSmithKline (GSK) Announces Agreement to Acquire IDRx
On January 13, 2025, GlaxoSmithKline (GSK) announced an agreement to acquire IDRx, a Boston-based clinical-stage biopharmaceutical company focused on developing precision therapies for gastrointestinal stromal tumors (GISTs). The core of this acquisition is IDRX-42, a highly selective KIT tyrosine kinase inhibitor (TKI) designed to treat GIST and target all key primary and secondary KIT mutations, addressing an unmet need in current treatments. GSK will pay up to $1.15 billion, including $1 billion in upfront payments and potentially an additional $150 million contingent upon successful regulatory approval.
IDRX-42 is considered a best-in-class drug due to its broad coverage of KIT mutations in GIST patients and its potential to improve tolerance, overcoming the limitations of existing therapies in terms of recurrence and resistance. Preliminary clinical trials have shown promising antitumor activity and manageable safety in advanced GIST patients, particularly in those receiving second-line or later treatments, with an objective response rate of 29%. Through this acquisition, GSK not only strengthens its portfolio in the gastrointestinal cancer space but also reaffirms its commitment to addressing significant unmet medical needs.
This acquisition reflects GSK's strategic goal of identifying and acquiring potential optimal molecules with targeted mechanisms of action to support the company’s long-term growth objectives through 2031 and beyond. The addition of IDRx brings advanced research and development capabilities, particularly in the area of developing efficient and selective targeted therapies. Additionally, IDRx’s other investors and supporters, including Andreessen Horowitz and Casdin Capital, further validate the company's technical expertise and market potential. This partnership is expected to further advance the development of treatments for GIST and other related cancers.
7.Bausch + Lomb Corporation's Subsidiary Completes Acquisition of Whitecap Biosciences, LLC
On January 13, 2025, Bausch + Lomb Corporation announced that its subsidiary had completed the acquisition of Whitecap Biosciences, LLC to enhance its research and development capabilities in the ophthalmic treatment field. The acquisition focuses on two innovative therapies currently in development for glaucoma and geographic atrophy (GA), both significant causes of severe vision loss.
Through this acquisition, Bausch + Lomb gains Whitecap Biosciences' efficient α-2 adrenergic agonist WB007, which has successfully completed Phase II clinical trials for the treatment of glaucoma, with plans for further clinical research targeting both glaucoma and GA. While the specific terms of the transaction were not disclosed, Bausch + Lomb has indicated that the acquisition will help address unmet medical needs and significantly improve current care standards, especially in slowing or even reversing vision loss caused by glaucoma.
Whitecap Biosciences, since its founding in 2015, has focused on developing new therapies for severe eye diseases, including glaucoma and GA. Dr. Scott Whitcup, founder of Whitecap Biosciences, emphasized the importance of partnering with Bausch + Lomb, believing it will accelerate the development of these promising therapies and lead to better visual outcomes for patients. This strategic acquisition not only reflects Bausch + Lomb’s commitment to finding and providing advanced ophthalmic treatments but also strengthens its leadership position in the global eye care market.
8.Salarius Pharmaceuticals and Decoy Therapeutics Announce Definitive Merger Agreement
On January 13, 2025, Salarius Pharmaceuticals, Inc. and Decoy Therapeutics, Inc. announced a definitive merger agreement, under which Decoy will merge with a wholly owned subsidiary of Salarius, and the combined company will be named Decoy Therapeutics. The merger aims to accelerate the clinical development of peptide-drug conjugates (PDCs) utilizing Decoy’s IMP3ACT™ platform, which integrates machine learning (ML) and artificial intelligence (AI) tools to design and manufacture innovative therapies, particularly targeting respiratory viruses and cancer.
Decoy Therapeutics focuses on developing next-generation peptide-drug conjugates, with a product pipeline that includes therapies for respiratory infectious diseases and gastrointestinal oncology indications. Through this merger, Salarius’ oral small-molecule protein degrader SP-3164 will also be incorporated into highly targeted peptide-based proteolysis-targeting chimeras (PROTACs) candidates. Additionally, Salarius’ ongoing development of sellidemstat for hematologic cancer will continue to be supported, and its strategic alternatives will be evaluated. Although the specific transaction amount was not disclosed in the announcement, the merged company will be jointly led by senior executives from both companies, including Decoy’s CEO, Frederick “Rick” Pierce, and other core team members.
Following the merger, the new company’s board will be composed of Rick Pierce, Barbara Hibner, and three independent directors, reflecting the spirit of collaboration and planning for future growth. David Arthur, President and CEO of Salarius Pharmaceuticals, emphasized that after a comprehensive strategic review, they believe the merger with Decoy Therapeutics represents the best opportunity to create significant value, not only based on the science behind Decoy’s peptide-drug conjugate technology but also due to its strong management team. This merger marks an important milestone for shareholders and presents new possibilities to address unmet needs across various therapeutic areas.
9.Biogen Announces Plan to Acquire All Outstanding Shares of Sage Therapeutics at $7.22 per Share
On January 10, 2025, Biogen announced its intention to acquire all of the outstanding shares of Sage Therapeutics at a price of $7.22 per share. This offer represents a premium of approximately 30% over Sage’s closing stock price at the time, with a total estimated valuation of approximately $469 million.
Upon receiving the proposal, the Sage board stated that it would carefully review and evaluate Biogen’s offer in consultation with its independent financial and legal advisors, in accordance with its fiduciary duties, to determine the course of action that is in the best interests of the company and all its shareholders. However, by January 27, 2025, the Sage Therapeutics board unanimously rejected Biogen’s non-binding acquisition proposal. Despite this, Sage’s stock price rose following the announcement, reflecting positive market sentiment toward the potential transaction.
It is noteworthy that Sage and Biogen have an existing collaboration, jointly developing and marketing Zurzuvae for the treatment of postpartum depression (PPD)/major depressive disorder (MDD) and SAGE-324 for essential tremor. This acquisition proposal could be part of Biogen’s strategy to further consolidate its position in the central nervous system (CNS) disease space. However, Sage believes that Biogen’s offer undervalues the company and may seek to maintain its independence to better develop its business. Furthermore, Sage has attempted to block Biogen’s acquisition efforts through legal means, indicating its strong opposition to the acquisition.
10.Veralox Therapeutics Announces Exclusive Agreement with Nudge Therapeutics
On January 9, 2025, clinical-stage biotechnology company Veralox Therapeutics announced an exclusive agreement with Nudge Therapeutics to acquire the latter and its preclinical series of cGAS (cyclic GMP-AMP synthase) inhibitors. This deal enables Veralox to leverage Nudge's novel small-molecule cGAS inhibitors, which are designed to prevent the overproduction of type I interferons associated with inflammation and autoimmune diseases, thus expanding its product portfolio.
While the specific financial terms were not disclosed in the announcement, the agreement stipulates that Veralox will be responsible for continuing the development of Nudge's cGAS inhibitors, with a comprehensive acquisition of Nudge Therapeutics upon reaching specific downstream milestones. One of Veralox's main focuses is VLX-1005, a new therapy targeting the 12-lipoxygenase (12-LOX) pathway, which is expected to achieve clinical proof of concept (PoC) by 2025. This transaction is intended to bolster Veralox's research and development capabilities in treating severe autoimmune and inflammatory diseases.
Robert Lowery, CEO of Nudge Therapeutics, emphasized the importance of Veralox's expertise in advancing the cGAS program, while Jonathan Mow, CEO of Veralox, noted that the potential applications of cGAS inhibitors span a range of inflammation-related diseases, including autoimmune, metabolic, cardiovascular, rare diseases, and neurodegenerative conditions, which align closely with Veralox's strategic goals. As such, this agreement not only expands Veralox’s product portfolio but also opens new possibilities for future drug development.
11.Vividion Therapeutics Announces Acquisition of Tavros Therapeutics
On January 8, 2025, Vividion Therapeutics announced its acquisition of Tavros Therapeutics, aiming to significantly enhance its functional genomics capabilities and further advance its drug discovery platform. This acquisition will enable Vividion to utilize Tavros' proprietary genomic screening methods to identify new drug targets and support the discovery and translational efforts for known targets, particularly in oncology.
Although the specific financial terms were not disclosed, this transaction will integrate Tavros' functional and computational genomics technologies into Vividion's platform, accelerating the development of novel therapeutic solutions. Vividion, a wholly owned subsidiary of Bayer, specializes in developing small-molecule therapies targeting high-value, hard-to-drug targets, and this acquisition further solidifies its position in precision oncology and immunology.
Notably, Bayer completed the full acquisition of Vividion for approximately $2 billion in August 2021, highlighting the interest of large pharmaceutical companies in small biotech firms with innovative technologies and potential. The acquisition of Tavros represents a significant step in expanding Vividion’s R&D capabilities and technological assets, reinforcing the trend of driving research progress through mergers and acquisitions, and its firm commitment to advancing new drug development.
12.Predictive Oncology Inc. Announces Binding Letter of Intent with Renovaro, Inc.
On January 6, 2025, Predictive Oncology Inc. announced that it has signed a binding letter of intent with Renovaro, Inc. to acquire Predictive Oncology by exchanging its shares for a newly created series of preferred stock issued by Renovaro. This transaction aims to combine the expertise of both companies in AI-driven drug discovery and multi-omics data processing to improve patient prognosis across various cancer indications while reducing operating costs by more than 30% in the short term.
Under the terms of the agreement, Predictive Oncology’s shareholders will receive Renovaro’s newly issued preferred stock on a 1:1 basis. These preferred shares can be converted into freely tradable common stock of Renovaro or redeemed under certain conditions. Renovaro must raise at least $15 million, and the transaction is subject to the formal approval of Predictive Oncology’s shareholders. If shareholder approval is not obtained, Renovaro will receive a two-year exclusive royalty-free license to Predictive Oncology's biorepository and related technologies as compensation.
This merger is expected to bring not only scientific synergies but also potentially enhance the combined company’s position in the capital markets and increase opportunities for business development. Both parties anticipate signing the final agreement no later than February 28, 2025, and the merged company will focus on accelerating drug discovery, reducing development risks, and providing more personalized treatment options for cancer patients.
Summary
January 2025 has been a transformative month for the biotechnology and pharmaceutical industries. Lantheus Holdings solidified its market position through two key acquisitions, strengthening its competitive edge in the treatment of neuroendocrine tumors, boosting its Alzheimer’s disease diagnostic market presence, and expanding its oncology product line. At the same time, other large pharmaceutical companies have actively participated in the M&A wave. Companies such as Johnson & Johnson, Eli Lilly, and GlaxoSmithKline (GSK) have expanded their product portfolios and technological capabilities through various acquisitions, such as J&J’s acquisition of Intra-Cellular Therapies and Eli Lilly’s investment in Scorpion Therapeutics. These moves significantly enhanced their strength in the fields of precision treatments for mental health and cancer.
With advances in technology and changes in market demand, M&A will continue to be a key strategy for companies to acquire new technologies, enter new markets, and address competition. It is expected that AI and digital technologies will play an increasingly important role in drug development, driving innovation and growth in the industry. Meanwhile, the recovery of capital markets may provide support for the growth of more small and mid-sized biotech companies, promoting the diversification of the entire industry. In response to evolving healthcare needs, integration and collaboration within the industry will help accelerate new drug development and improve the quality of medical services, offering more hope to patients worldwide.
In summary, the M&A activities in January 2025 highlight several key trends in the biopharmaceutical industry: First, enhancing core competitiveness and technological innovation through mergers and acquisitions; second, addressing unmet medical needs, particularly in neuroscience and oncology; and third, optimizing financial structures and improving business efficiency through restructuring and collaboration. These trends collectively drive industry progress and lay a solid foundation for future growth.
How to get the latest progress on drug deals?
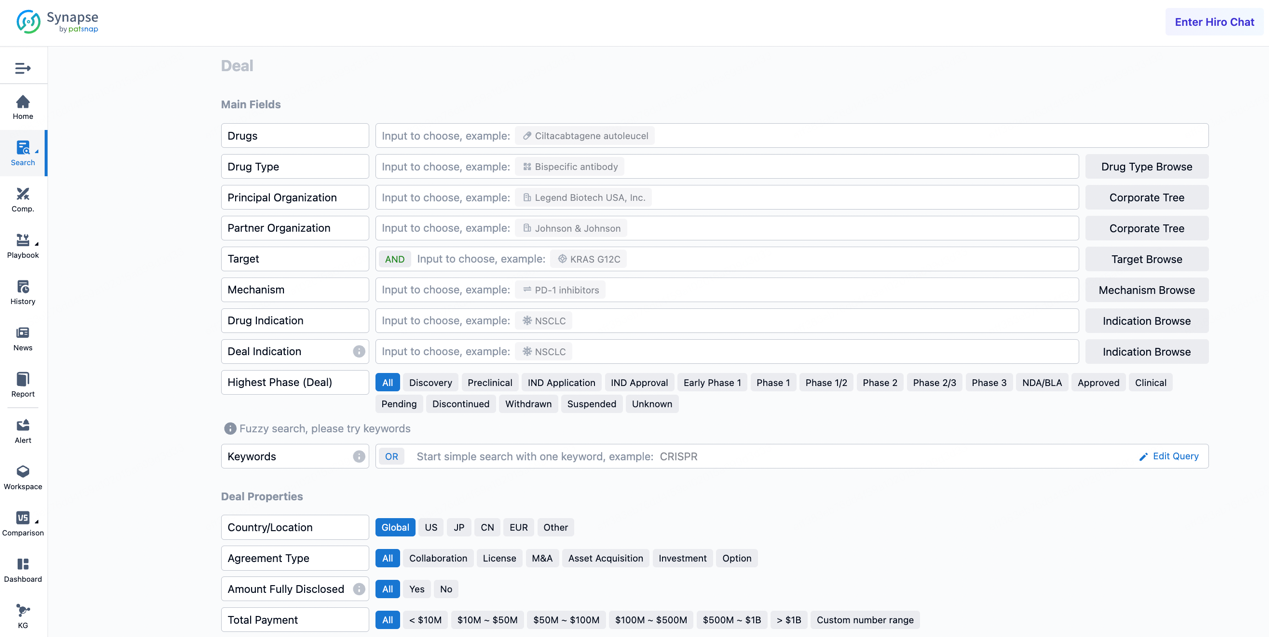
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
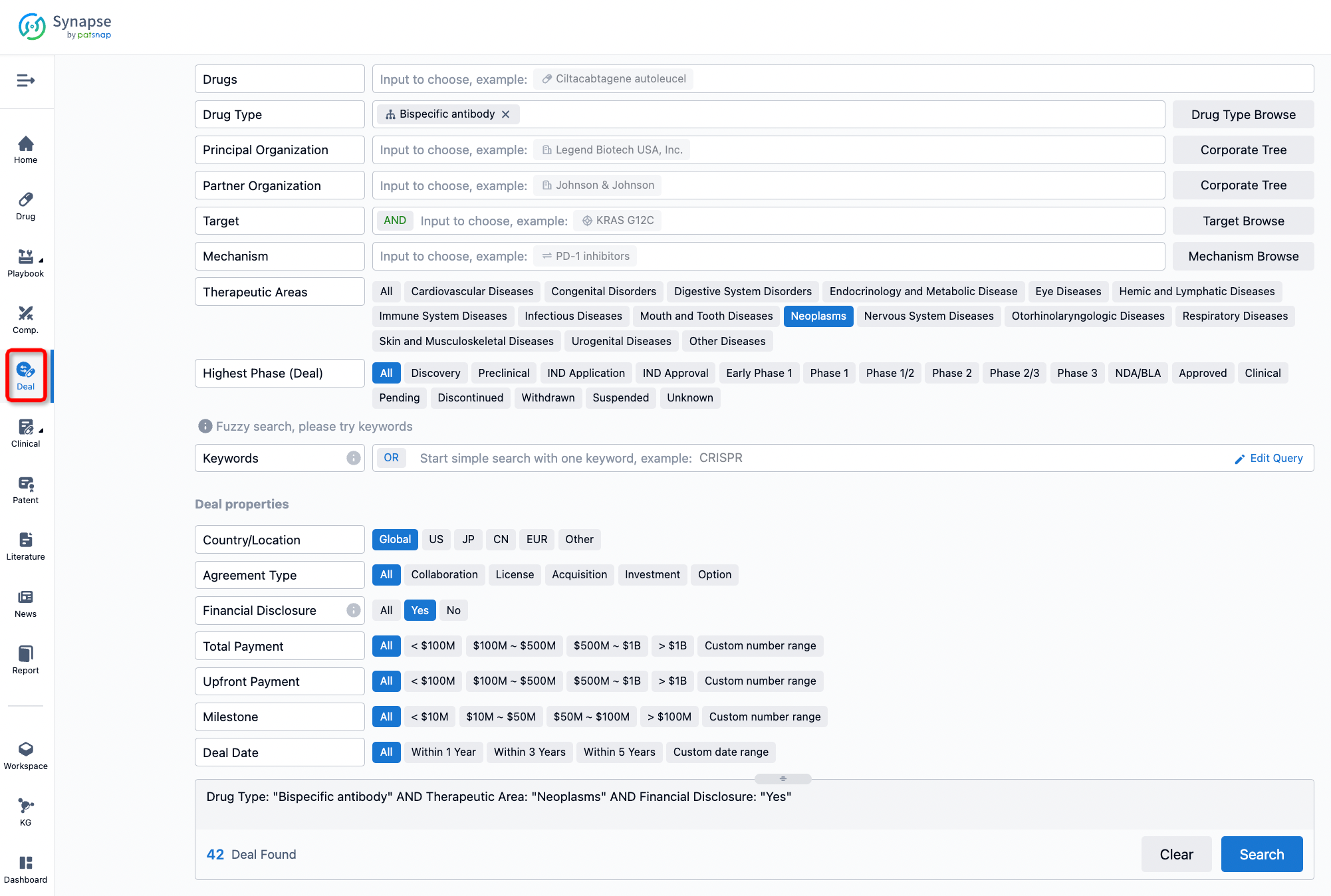
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
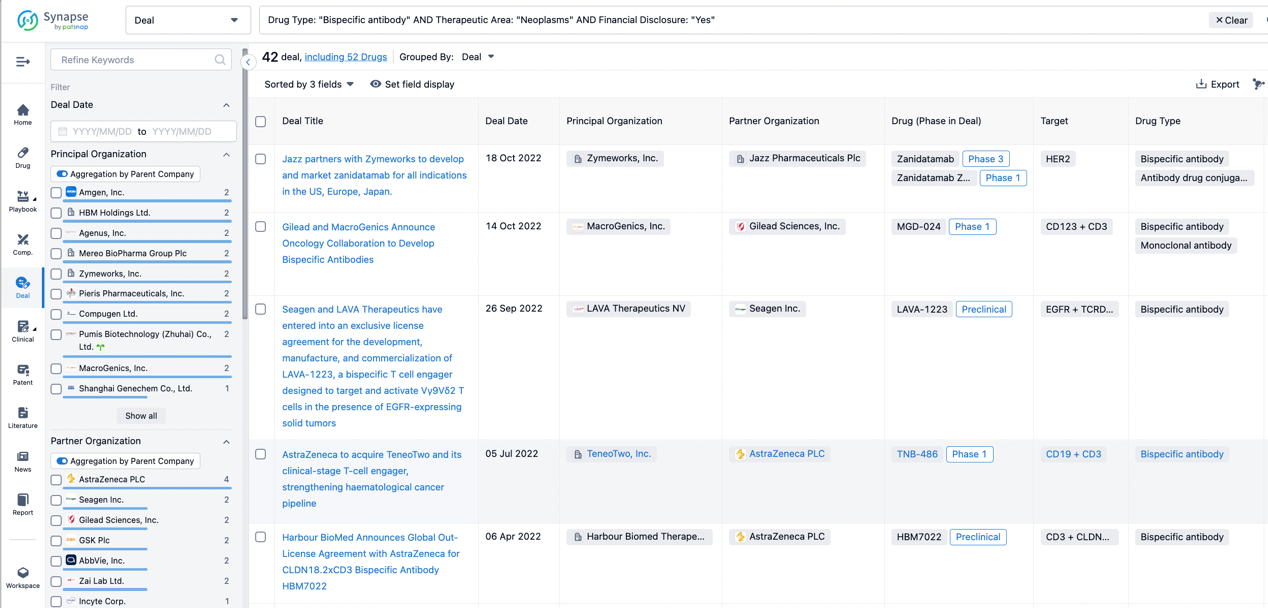
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
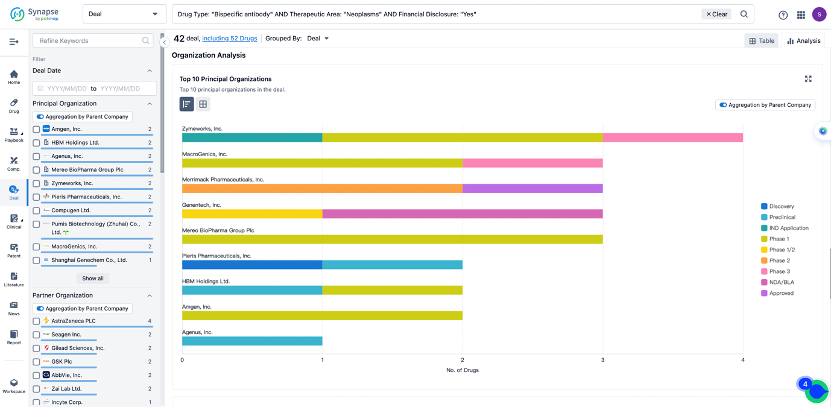
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.

In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
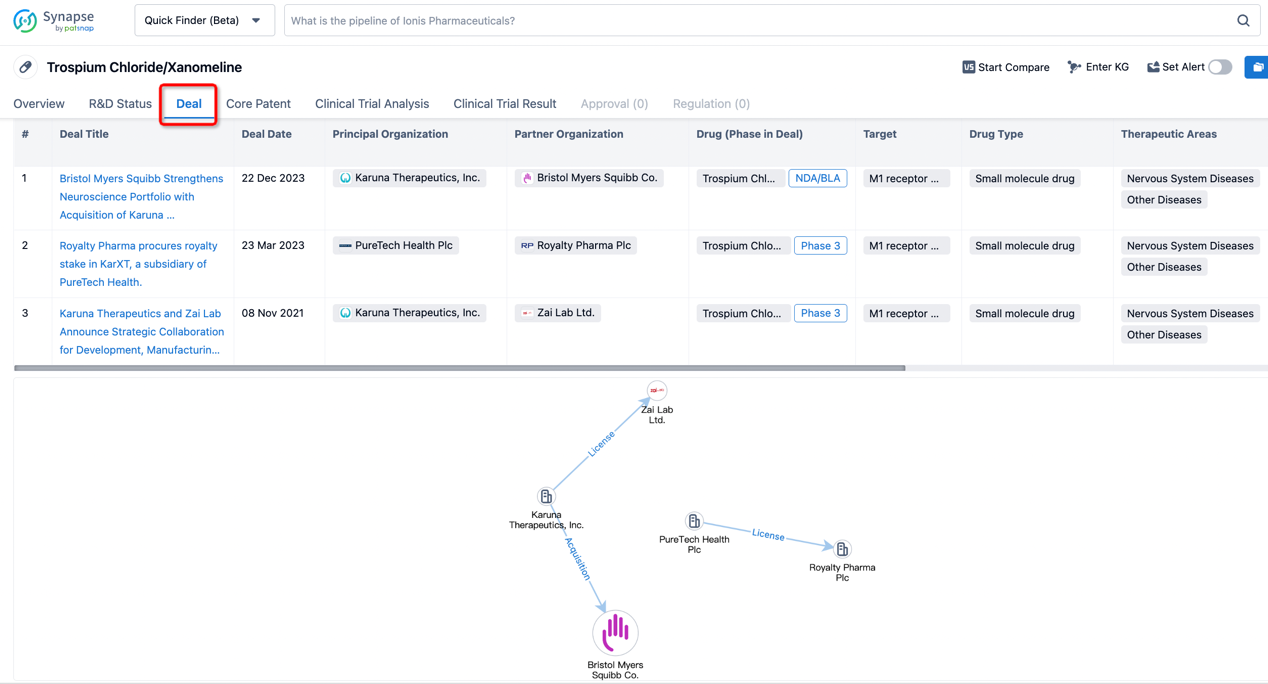
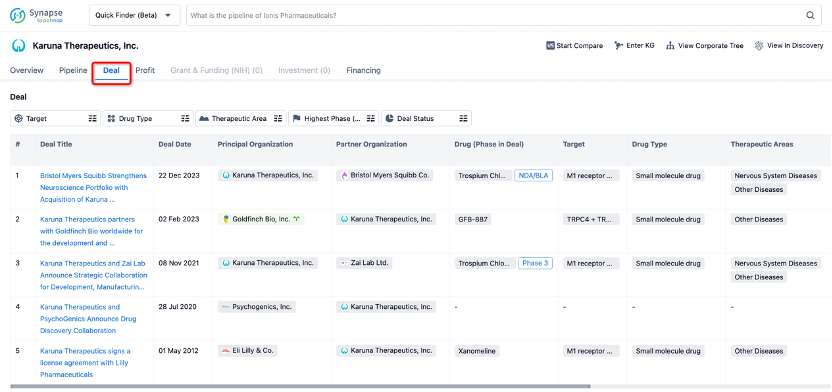
Click on the image below to explore new pharmaceutical funding transactions!