Kairos Pharma Starts Phase 1 Trial of ENV105 Plus Osimertinib in EGFR-Related Lung Cancer Treatment
Kairos Pharma, Ltd., an enterprise focused on the clinical phase development of new treatments targeting oncological conditions, has initiated the administration of its premiere dual regimen in a Phase 1 study. This trial involves a novel pairing of the company's primary investigational drug, ENV105, with the established medication osimertinib, aiming to tackle non-small cell lung cancer. This innovative approach is strategically crafted to combat the challenges of drug resistance and immunological inhibition in cancer care, marking a significant step forward with the treatment of the initial pair of participating patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
John Yu, the Chief Executive Officer at Kairos Pharma, commented, "Launching this clinical study underscores the commitment of our Kairos team to address current critical healthcare gaps through innovation in treatment."
The main goal of this non-blinded study is to assess the combined treatment's safety profile and its level of tolerability. The study's participant recruitment target includes 60 individuals who have either become resistant to osimertinib or have exhibited only a partial response to the medication.
Patients suffering from lung cancer with mutations in the epidermal growth factor receptor are typically treated with EGFR inhibitors such as osimertinib as an initial therapy.
However, over time, these same patients often exhibit a tolerance to osimertinib. ENV105 is a pioneering biotherapeutic created to improve the response of patients with EGFR-mediated lung cancer to such EGFR inhibitors.
Kairos Pharma's Chief Scientific Officer, Dr. Neil Bhowmick, further explained, "Incorporating ENV105 in the treatment regimen for patients who have shown resistance to EGFR inhibitors like osimertinib is a strategic move. This is because inhibiting EGFR triggers environmental variables of CD105, a critical component known to facilitate the advancement of tumors."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
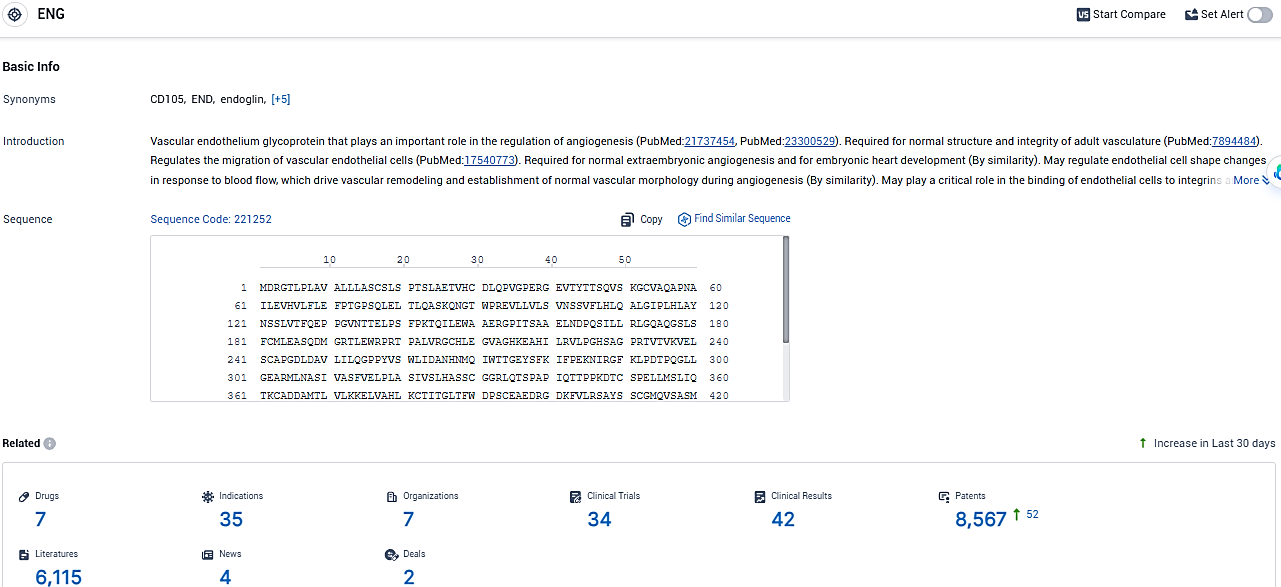
According to the data provided by the Synapse Database, As of January 24, 2024, there are 7 investigational drugs for the ENG target, including 35 indications, 7 R&D institutions involved, with related clinical trials reaching 34, and as many as 8567 patents.
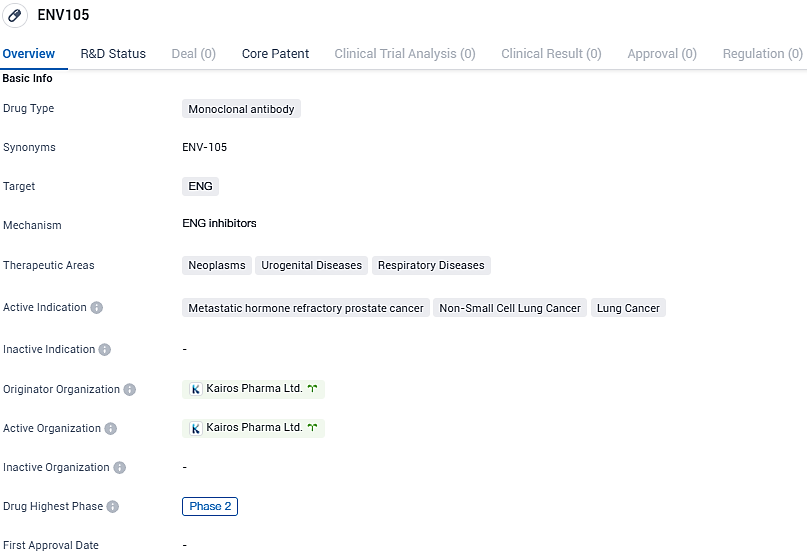
ENV105 is a monoclonal antibody drug that targets ENG and is being developed for the treatment of neoplasms, urogenital diseases, and respiratory diseases. It has shown promise in the treatment of metastatic hormone refractory prostate cancer, non-small cell lung cancer, and lung cancer. Kairos Pharma Ltd. is the originator organization behind the drug, and it is currently in Phase 2 of development.