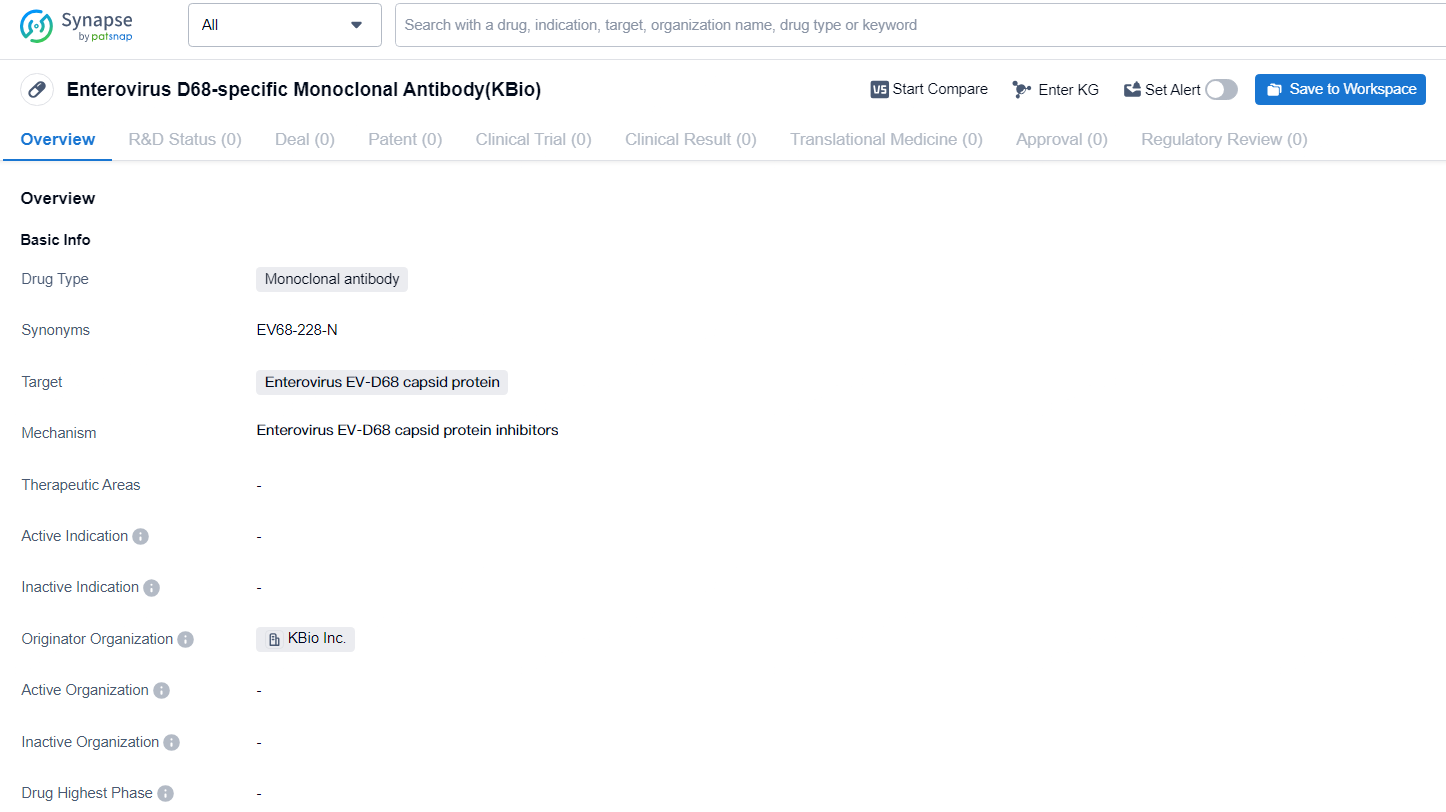
KBio's EV68-228-N Approved by FDA for Acute Flaccid Myelitis Treatment, Initiates Phase 1 Trial
KBio, a top company in creating innovative biological therapies utilizing a plant-derived production system, has today revealed that the investigational new drug application for EV68-228-N has received clearance from the United States Food and Drug Administration. EV68-228-N is a human monoclonal IgG1 targeting the capsid of enterovirus D68 (EV-D68) and is intended for intravenous administration to treat acute flaccid myelitis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
EV68-228-N is produced by KBio through its innovative Rapid Protein Production Platform, utilizing Nicotiana benthamiana for biologics manufacturing. The Drug Master File submitted by KBio underwent FDA review as part of the IND application.
The Phase 1 clinical trial, which is placebo-controlled and double-blinded, aims to assess the safety and pharmacokinetics of incrementing single doses of EV68-228-N administered intravenously to healthy adult subjects. The initial participant received the dose at Vanderbilt University Medical Center.
The discovery of EV68-228 was made by Dr. Matthew Vogt during his postdoctoral tenure in the Crowe lab at Vanderbilt University Medical Center. Collaborating with Dr. Vogt and Dr. C. Buddy Creech—principal investigator of the study and director of the Vanderbilt Vaccine Research Program—KBio and ZabBio effectively transitioned the antibody from research to its inaugural human trial. They utilized the RP3 platform and combined their extensive regulatory experience in plant-based biopharmaceutical development.
"We are delighted that the FDA has approved the IND for EV68-228-N for use in Acute Flaccid Myelitis (AFM), a rare and severe neurological disorder that leads to muscle weakness and progressive paralysis. With AFM cases increasing since 2014 and no approved treatments available, this marks an important step forward," stated Patrick Doyle, CEO of KBio.
Monoclonal antibodies hold vast potential across various therapeutic fields, but challenges in their production can limit their life-saving impact. By adopting an environmentally friendly production system that provides unmatched speed and scalability, KBio aims to develop monoclonal antibody treatments and vaccines that could address a wide spectrum of medical conditions and public health issues.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
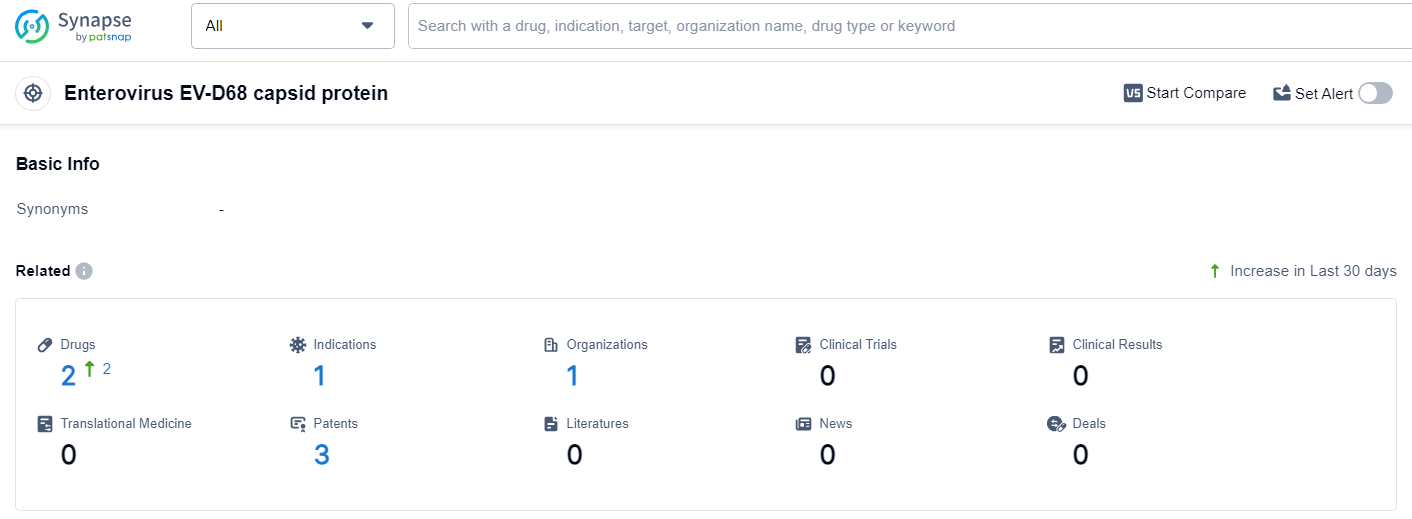
According to the data provided by the Synapse Database, As of July 3, 2024, there are 2 investigational drugs for the Enterovirus EV-D68 capsid protein target, including 1 indication, 1 R&D institution involved, and as many as 3 patents.
EV68-228-N represents a potential advancement in the field of biomedicine, particularly in the treatment of Enterovirus EV-D68 infections. As a targeted therapy, monoclonal antibodies have the potential to provide a specific and effective treatment option for individuals affected by Enterovirus EV-D68. However, further research and development, as well as regulatory approval, may be necessary before this drug becomes widely available for clinical use.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!