Kineta Reports Encouraging Findings for KVA12123 Monotherapy from Phase 1/2 Clinical Study
Clinical-stage biotech firm Kineta, Inc., engaged in pioneering unique immunotherapies in the oncology realm to tackle cancer immune resistance, has provided new insights on their progressing Phase 1/2 clinical study of KVA12123 for patients afflicted with advanced solid tumors.
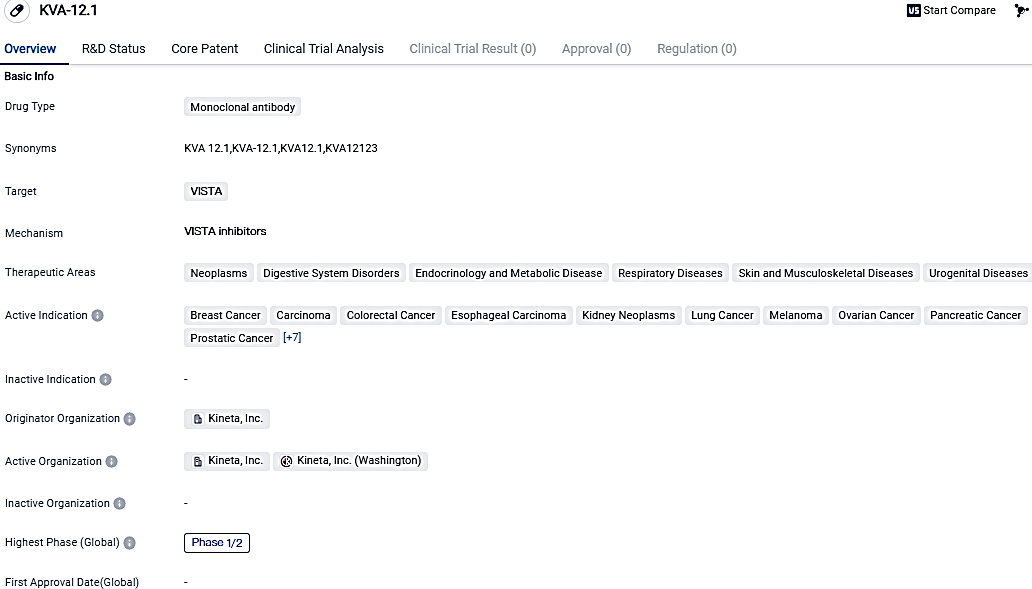
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Kineta's immuno-oncology medication, KVA12123, which is pointed at VISTA, passed the initial three monotherapy dosage levels and was demonstrated to be safe, with no dose limiting toxicities or cytokine-related harmful events detected. Furthermore, KVA12123 showed a pharmacokinetic profile that exceeded dose-proportional, achieving over 90% VISTA receptor occupancy among patients in the 30 mg dosing group.
Shawn Iadonato, Ph.D., the Chief Executive Officer of Kineta, expressed satisfaction with the progression of the Phase 1/2 clinical trial. He highlighted the promising preliminary safety and pharmacokinetic findings for KVA12123, which they believe substantially reduces the risk of VISTA as a novel target for drugs. He showed gratitude for the participation of patients and healthcare providers, and looks forward to further explore the potential of KVA12123 for the treatment of advanced solid tumors.
The VISTA-101 trial included 11 patients with advanced solid tumors from the first three monotherapy dosage-increasing cohorts, where participants received 3, 10, or 30 mg of KVA12123 through intravenous infusion every fortnight. The primary goals of the Phase 1/2 study are to assess the safety and tolerance of KVA12123 and establish the recommended dosage for Phase 2. Those enrolled in the study all had extensive previous treatment including chemotherapy, radiation, and immunotherapy.
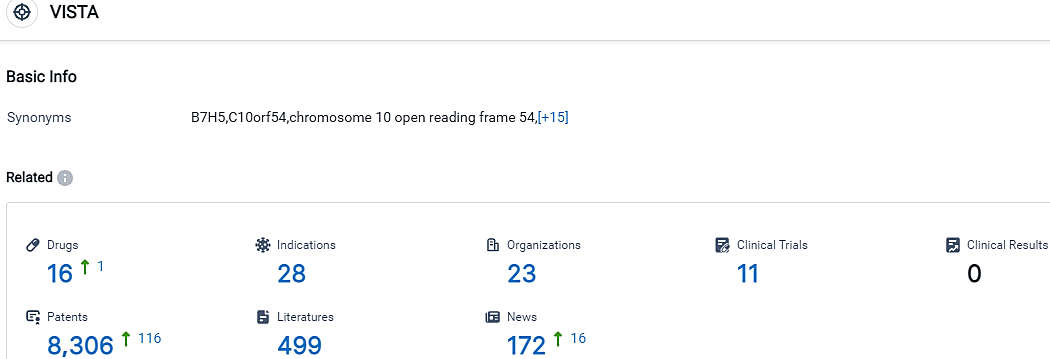
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 14, 2023, there are 16 investigational drugs for the VISTA target, including 28 indications, 23 R&D institutions involved, with related clinical trials reaching 11,and as many as 8306 patents.
KVA12123 has potential applications in various therapeutic areas, including cancer and other diseases affecting the digestive system, endocrine system, respiratory system, skin, musculoskeletal system, and urogenital system.