Kyverna Therapeutics gets FDA RMAT status for KYV-101 to treat progressive myasthenia gravis
Kyverna Therapeutics, Inc. (Kyverna), a clinical-stage biopharmaceutical firm prioritizing patient-centered care and specializing in the creation of cell therapies for autoimmune disease patients, revealed today that the U.S. Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to its autologous, fully human CD19 chimeric antigen receptor (CAR) T-cell product candidate, KYV-101, aimed at treating individuals with progressive myasthenia gravis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 "The RMAT designation highlights the FDA's focus and interest in advancing groundbreaking therapies for severe autoimmune diseases like myasthenia gravis," stated Srikanth Muppidi, M.D., a neuromuscular disorder expert at Stanford Medicine in Palo Alto, CA, and the primary investigator in the KYSA-6 trial. "We are in a period of significant evolution in the treatment approach for autoimmune conditions, and we aspire towards achieving a symptom-free state for patients."
"The RMAT designation highlights the FDA's focus and interest in advancing groundbreaking therapies for severe autoimmune diseases like myasthenia gravis," stated Srikanth Muppidi, M.D., a neuromuscular disorder expert at Stanford Medicine in Palo Alto, CA, and the primary investigator in the KYSA-6 trial. "We are in a period of significant evolution in the treatment approach for autoimmune conditions, and we aspire towards achieving a symptom-free state for patients."
"We are very pleased with the positive scientific relationship that has been formed between Kyverna and the FDA," commented Peter Maag, Ph.D., CEO of Kyverna. "We are confident that the RMAT designation will enhance our diligent efforts in developing KYV-101, with the aim of aiding the most deserving patients."
About Myasthenia Gravis (MG)
Myasthenia gravis is an autoimmune condition that leads to muscle weakness across various parts of the body. This can result in partial eye movement paralysis, difficulties in chewing and swallowing, respiratory issues, speech problems, and general skeletal muscle weakness. Patients with MG develop antibodies that cause an immune attack on essential signaling proteins at the neuromuscular junction, impairing nerve-to-muscle communication. Early-stage symptoms can be transient and may remit spontaneously, but as the disease advances, symptom-free intervals decrease and exacerbations can last for months. Approximately 80% of patients reach peak disease severity within two to three years, and up to 20% of MG patients face respiratory crisis at least once in their lifetime.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
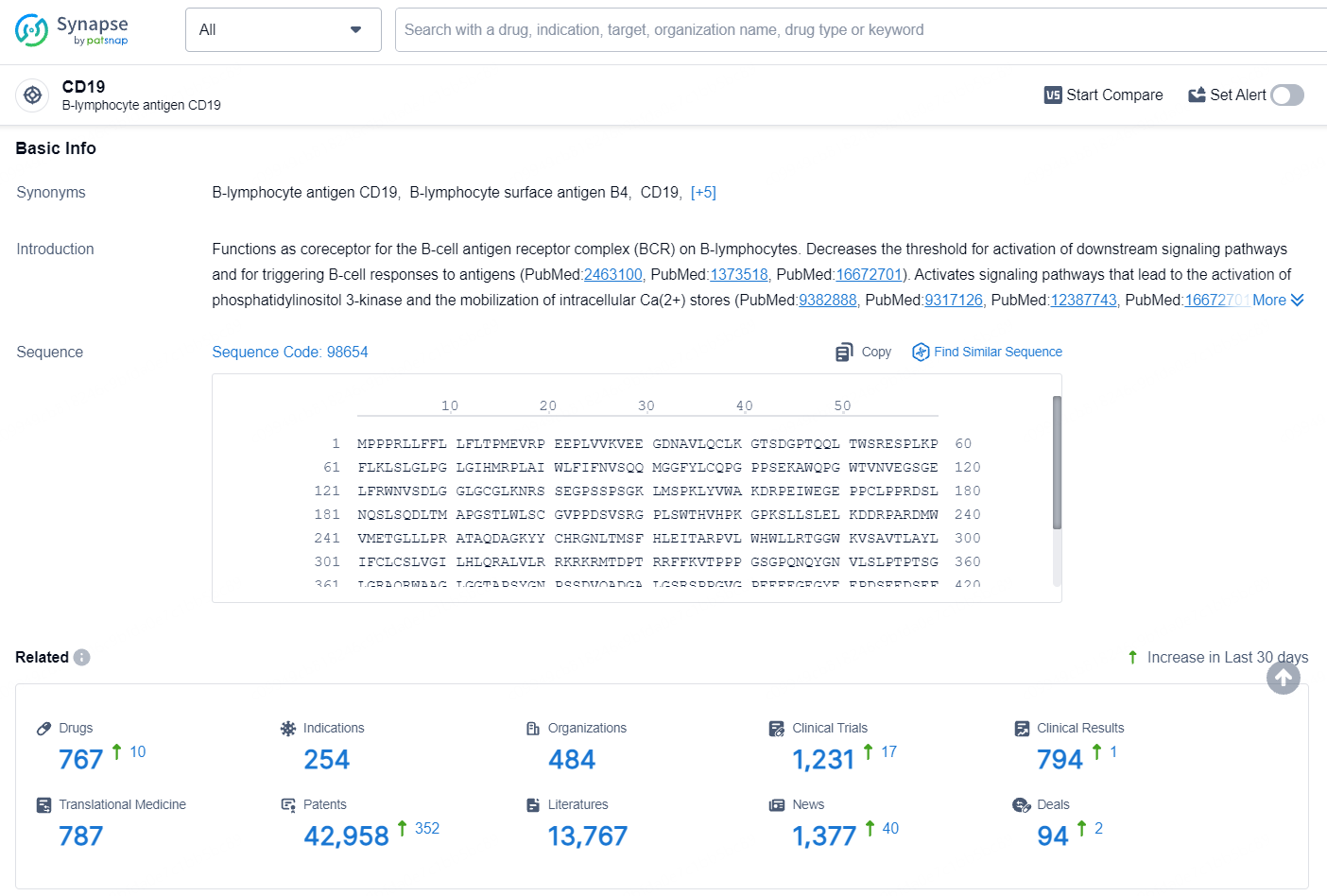
According to the data provided by the Synapse Database, As of August 14, 2024, there are 16 investigational drugs for the CD19 target, including 254 indications, 484 R&D institutions involved, with related clinical trials reaching 1231, and as many as 42958 patents.
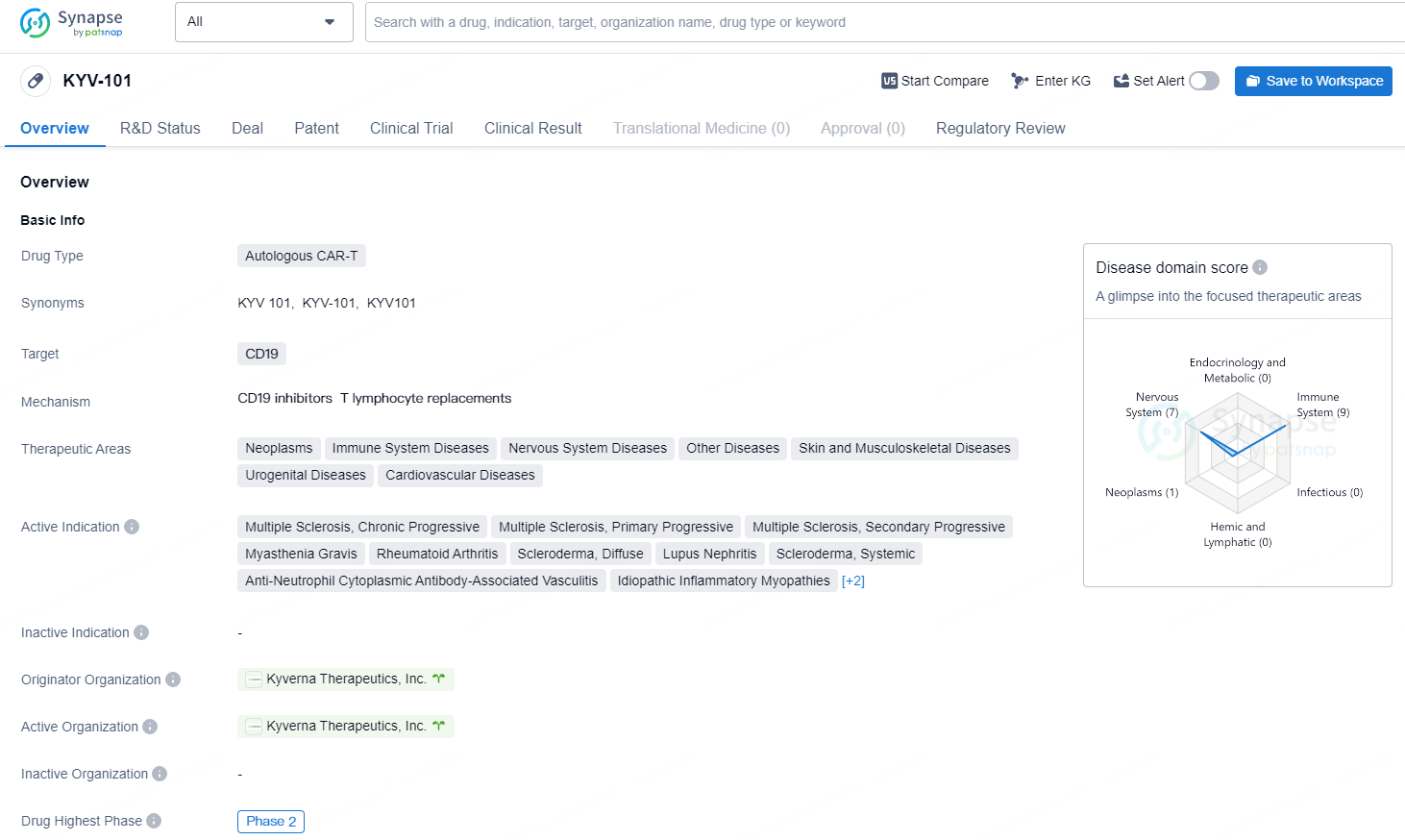
KYV-101 is an autologous, fully human CD19 CAR T-cell product candidate for use in B cell-driven autoimmune diseases. The CAR in KYV-101 was designed by the National Institutes of Health (NIH) to improve tolerability and tested in a 20-patient Phase 1 trial in oncology. Results were published by the NIH in Nature Medicine.KYV-101 is currently being evaluated in sponsored, open-label, Phase 1/2 and Phase 2 trials of KYV-101 in the United States and Germany across two broad areas of autoimmune disease: rheumatology and neurology.