LAPIX Therapeutics Announces Positive Phase 1 Trial Results for Autoimmune Treatment LPX-TI641
LAPIX Therapeutics, Inc. (“LAPIX”), a biopharmaceutical firm in the clinical stage that specializes in creating innovative orally available therapies aimed at restoring the immune system for autoimmune conditions through TIM (T cell/transmembrane, immunoglobulin, and mucin receptor) agonism, has reported favorable topline findings from its Phase I randomized, double-blind, placebo-controlled trial involving single and multiple ascending doses (SAD and MAD). This study evaluated the safety, tolerability, and pharmacokinetics (PK) of LPX-TI641 following oral administration in healthy adult subjects, designated as LPX641-101 (NCT05853835).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The results from the Phase 1 clinical trial indicated that LPX-TI641 was safe and tolerated well at the tested oral doses, which ranged from 10 mg to 150 mg in the Single Ascending Dose (SAD) segment and from 30 mg to 120 mg once daily for up to 7 days in the Multiple Ascending Dose (MAD) part. No maximum tolerated dose was determined during this investigation. Adverse effects reported were mainly mild and occurred sporadically in both the placebo and treatment cohorts, with no correlation to the dosage levels. The most frequently reported adverse effect was mild headaches. Importantly, treatment-related neutropenia and lymphocytopenia—two typical side effects of autoimmune treatments—were not found in participants receiving differing doses of LPX-TI641.
“We are dedicated to addressing the complexities of the immune system,” stated Anas M. Fathallah, Ph.D., co-founder and CEO of LAPIX. “The newly emerging preliminary data, especially the observed exposure-dependent alterations in our key exploratory biomarkers, T-regs and B-regs, underscore LPX-TI641’s potential as a leading treatment for autoimmune disorders. We are keen to move this promising candidate into a Phase Ib trial involving rheumatoid arthritis (RA) and psoriatic arthritis (PsA) to potentially establish a new oral standard of care for those affected by autoimmune diseases.”
LPX-TI641 exhibited oral bioavailability with a dose-dependent rise in exposure with increased dosing. Initial exploratory pharmacodynamic results from the trial showed a statistically significant rise in circulating CD4+/Foxp3+ T-cells (T-regs) and CD25+/CD19+ B-cells (B-regs) in correlation with LPX-TI641 exposure compared to the pooled placebo group.
The Phase I clinical trial included six cohorts for SAD and three for MAD. A total of 72 participants (eight per cohort, comprising six on active treatment and two on placebo) were enrolled consecutively. Out of the 72 individuals, 70 completed all required study activities and visits, while two participants withdrew for personal reasons unrelated to any drug-related adverse effects.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
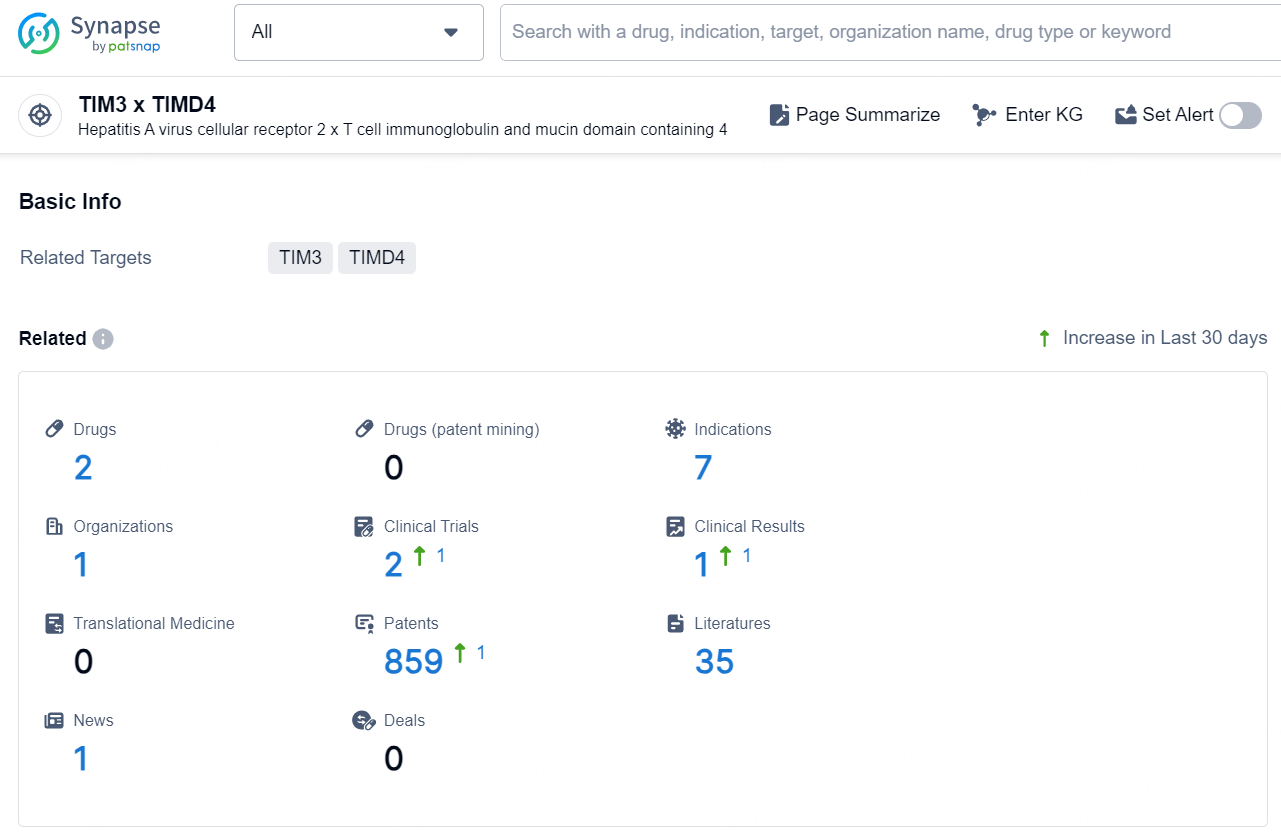
According to the data provided by the Synapse Chemical, As of November 26, 2024, there are 2 investigational drugs for the TIM3 and TIMD4 target, including 7 indications, 1 R&D institution involved, with related clinical trials reaching 2, and as many as 859 patents.
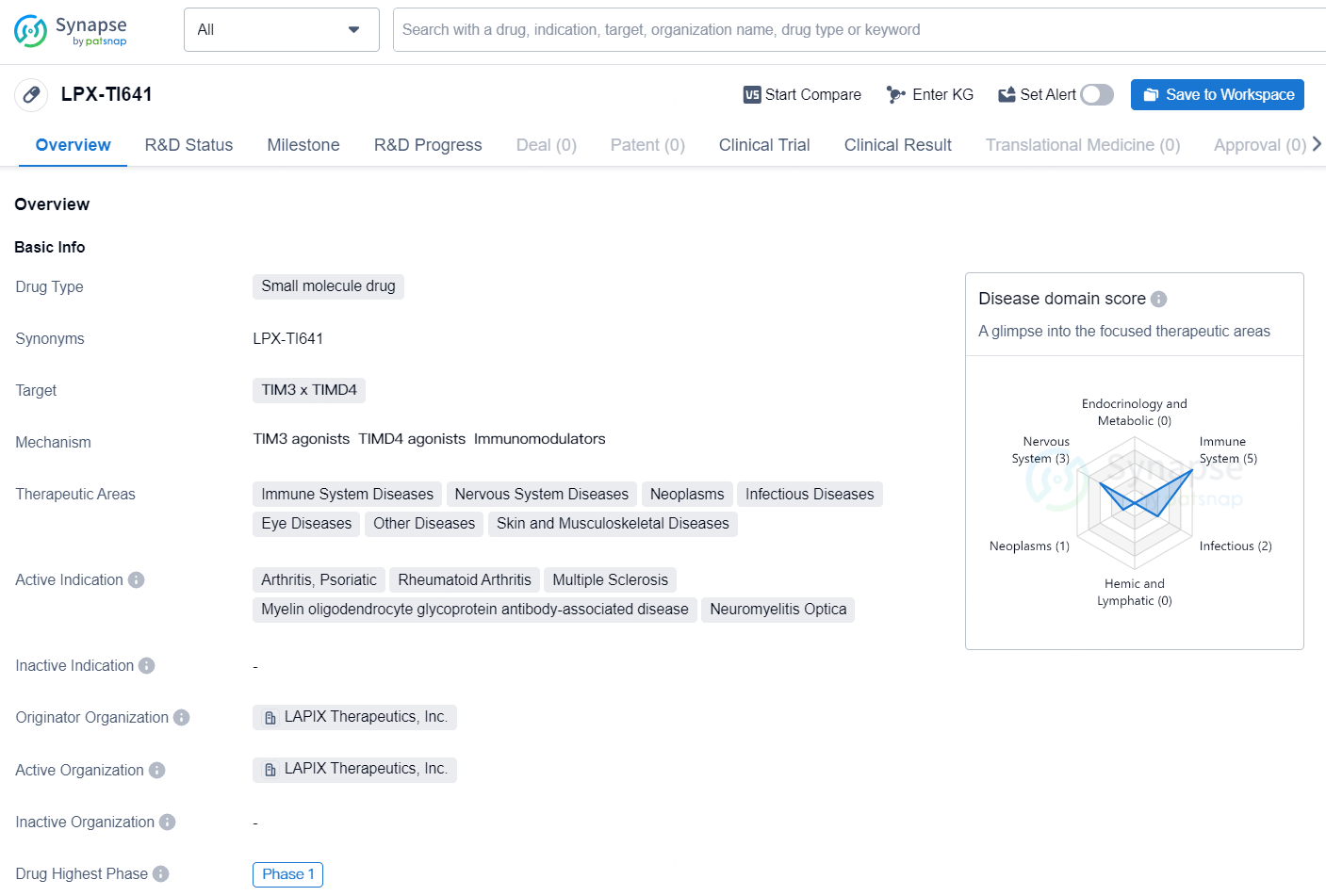
LPX-TI641 is a small molecule drug developed by LAPIX Therapeutics, Inc. The drug targets the proteins TIM3 and TIMD4 and is intended to treat a wide range of therapeutic areas, including immune system diseases, nervous system diseases, neoplasms, infectious diseases, eye diseases, skin and musculoskeletal diseases, and other diseases. LPX-TI641 is currently in the highest global phase of development, Phase 1.