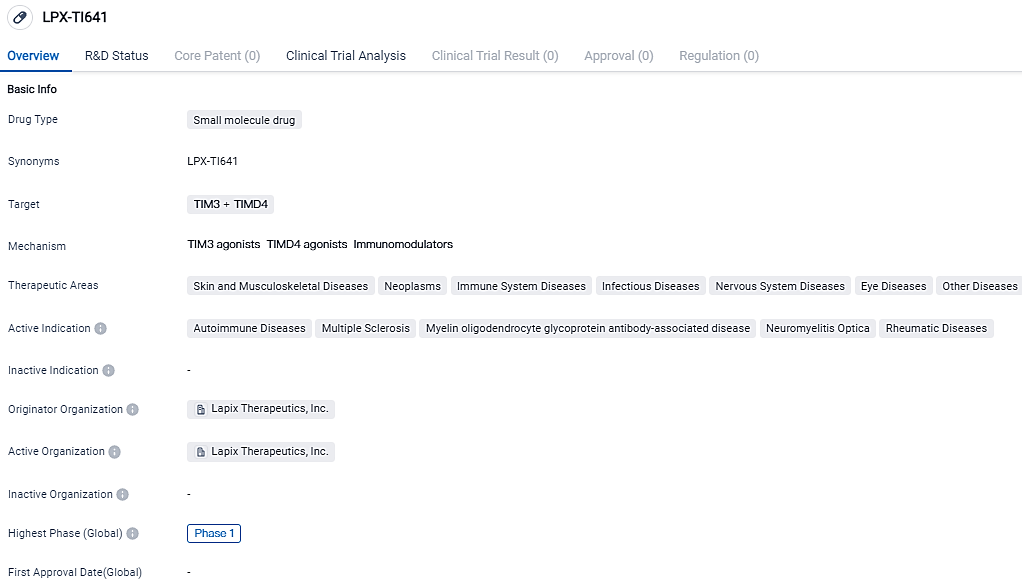
LAPIX Therapeutics Inc. reports FDA has approved their IND application for LPX-TI641, designed to treat for Multiple Sclerosis
LAPIX Therapeutics, Inc., a biopharmaceutical firm dedicated to the creation of ground-breaking orally administered therapies to rejuvenate the immune system for autoimmune ailments, divulged the clearance of its IND application by the U.S. FDA. The clearance allows the initiation of Phase 1 clinical trial for their trailblazer, immune tolerance restoring small compound, LPX-TI641, intended for multiple sclerosis treatments. The clinical trial is projected to initiate the medication procedure in 4Q 2023.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
" We believe in empowering and restoring the immune system in its battle against diseases. FDA's clearance for our IND application for LPX-TI641 symbolizes a forward-direction for our technological and scientific methodology," stated Anas M. Fathallah, Ph.D., CEO and co-inventor of LAPIX. "Our enthusiasm is buoyed for initiating the foremost human trial to assess LPX-TI641, potentially spearheading a unique, non-immune suppressive therapy for autoimmune afflictions."
The initial phase of the trial is set to gauge the safety, tolerance, and pharmacokinetics of LPX-TI641 in healthy individuals post a singular escalating dosage. Information garnered from this initial stage coupled with LPX-TI641's antigen-agnostic flexibility might allow for the rapid pivot to other autoimmune indications such as rheumatoid arthritis.
Dr. Fathallah further emphasized the aspiration to revolutionize the way serious and intricate immune diseases are treated in the medical sector. "We are adamantly focused on extending help to the maximum possible extent. Our comprehensive understanding of immunology and immune resilience, and our approach, which lays focus on the development of Tim agonists for restoring antigen-agnostic immune tolerance is at the crux of developing potentially life-changing treatments for patients, while also being considerate of their lifestyle."
LPX-TI641 is a pioneering investigational, and exclusive orally bioavailable small molecular T cell immunoglobulin and mucin domain-containing protein (TIM) 3/4 receptor agonist. The TIM receptor family has a pertinent role in autoimmunity. Among LPX-TI641's initial pharmacological actions are the intensification of Foxp3+/DC4+ T-cells and Tim1+/CD25+/CD19+ and the restraint/downgrading of Th17.
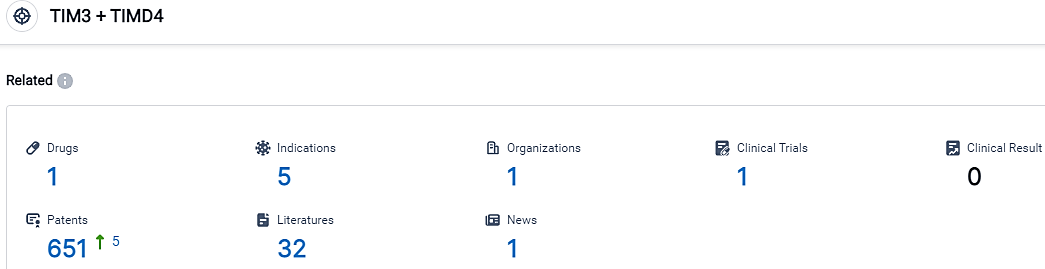
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 25, 2023, there are 1 investigational drugs for the TIM3 and TIMD4 target, including 5 indications,1 R&D institutions involved, with related clinical trials reaching 1,and as many as 651 patents.
The efficacy of LPX-TI641 versus standards of care has been evaluated in several animal models of MS and those models have shown favorable efficacy of LPX-TI641 in treatment escalation. It is currently under development for neuro-autoimmune indications such as MS, with the intent to expand into rheumatoid arthritis amongst other autoimmune conditions.