Latest Competitive Analysis of Johnson & Johnson Drug Pipeline
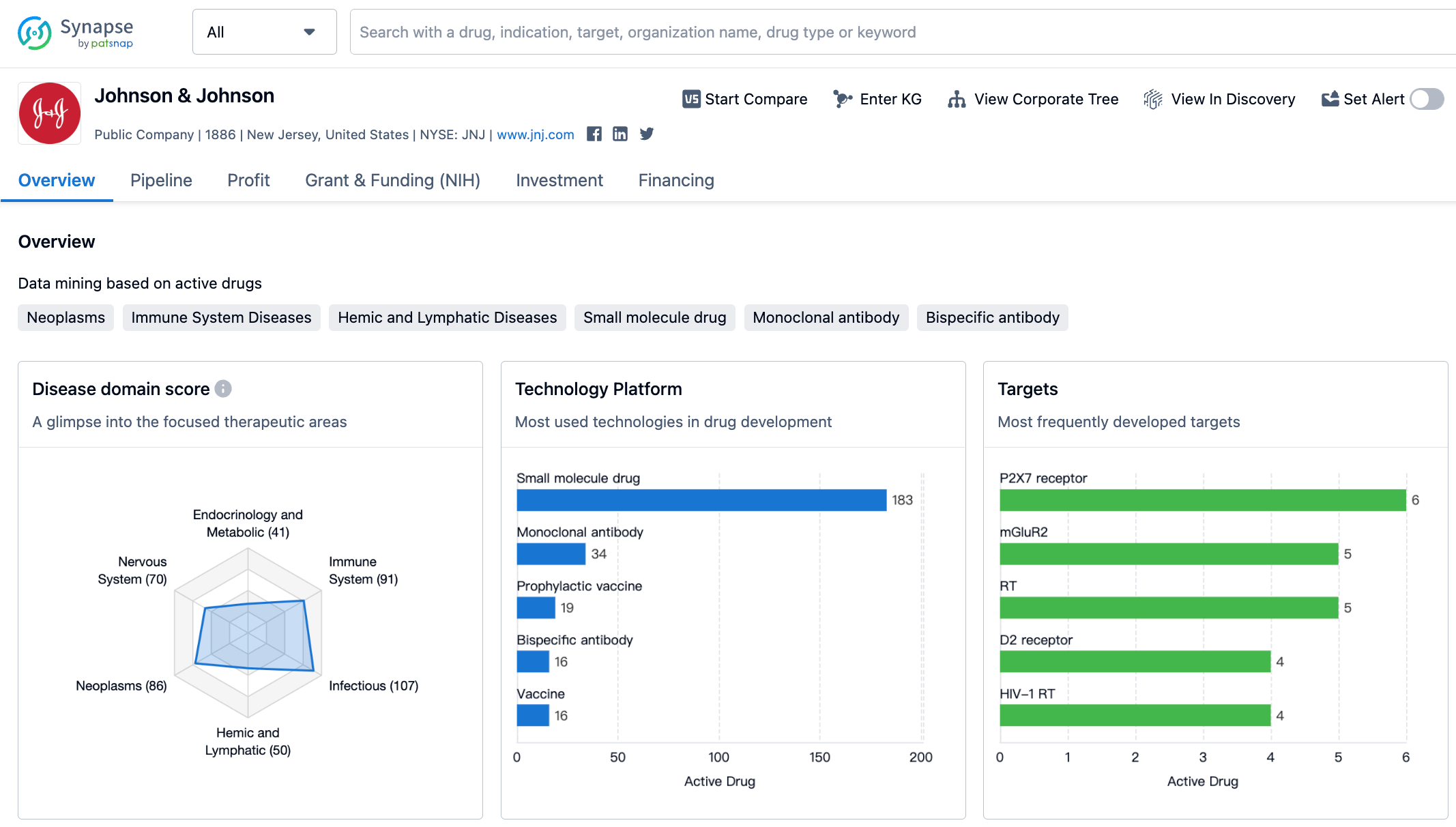
The performance of Johnson & Johnson's pharmaceutical sector is quite robust among large pharmaceutical companies, with the driving force of the two major engines - Ustekinumab and Daratumumab - being very adequate. Its PAH line also performed well. However, former heavyweight products such as Ibrutinib, Infliximab, and Abiraterone are indeed showing signs of aging.
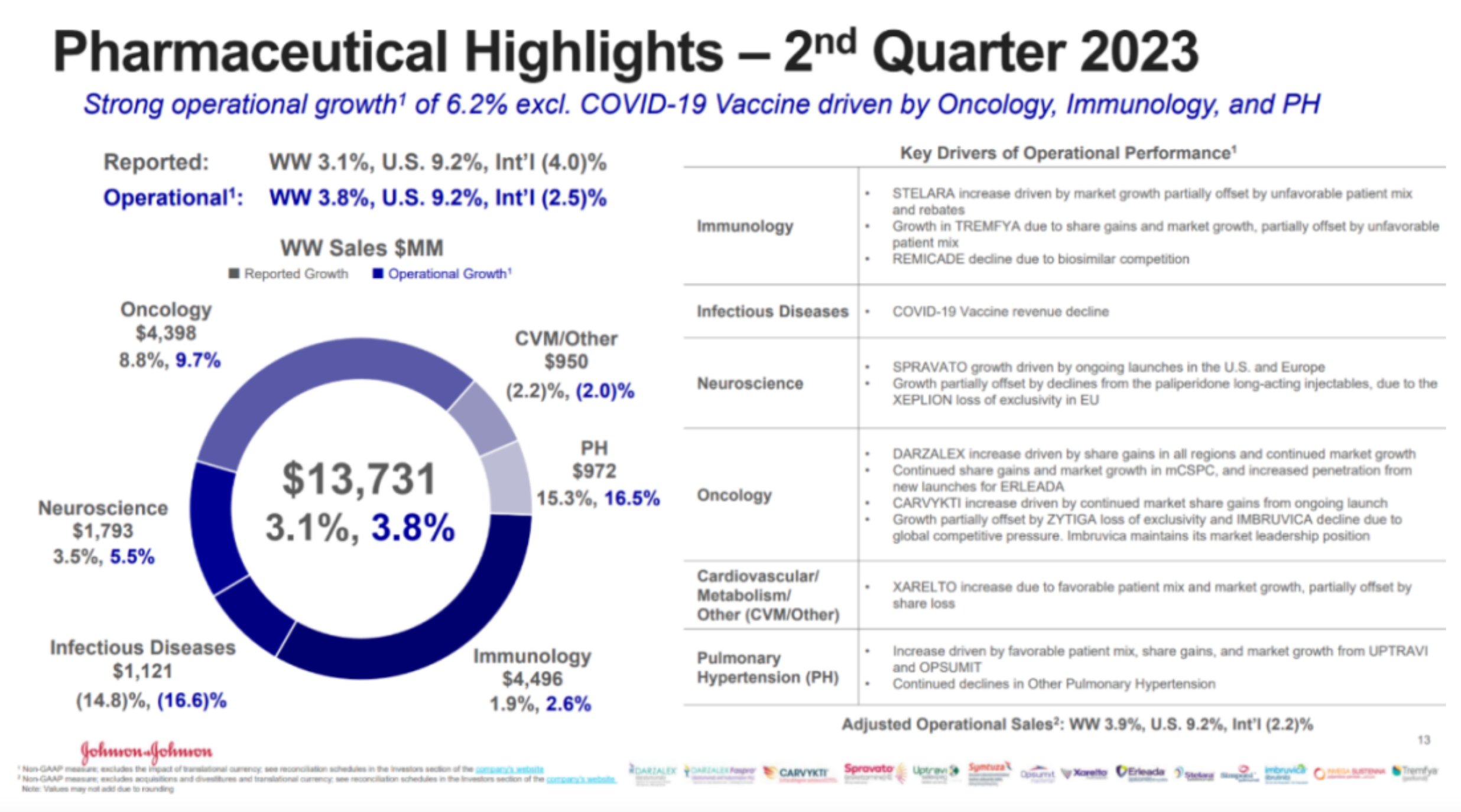
The most outstanding progress in the research pipeline is still the "blocking" advantage in the field of multiple myeloma, including the legendary Cilta-cel announcing second-line MM data and submitting sBLA, GPRC5D/CD3 bispecific antibody Talquetamab getting approval, and the already approved CD3/BCMA bispecific antibody Teclistamab announcing long-term follow-up data.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Johnson & Johnson.
This pipeline is expected to maintain J&J's monopoly in this field in the long term behind CD38 and become the core support for future years' performance.
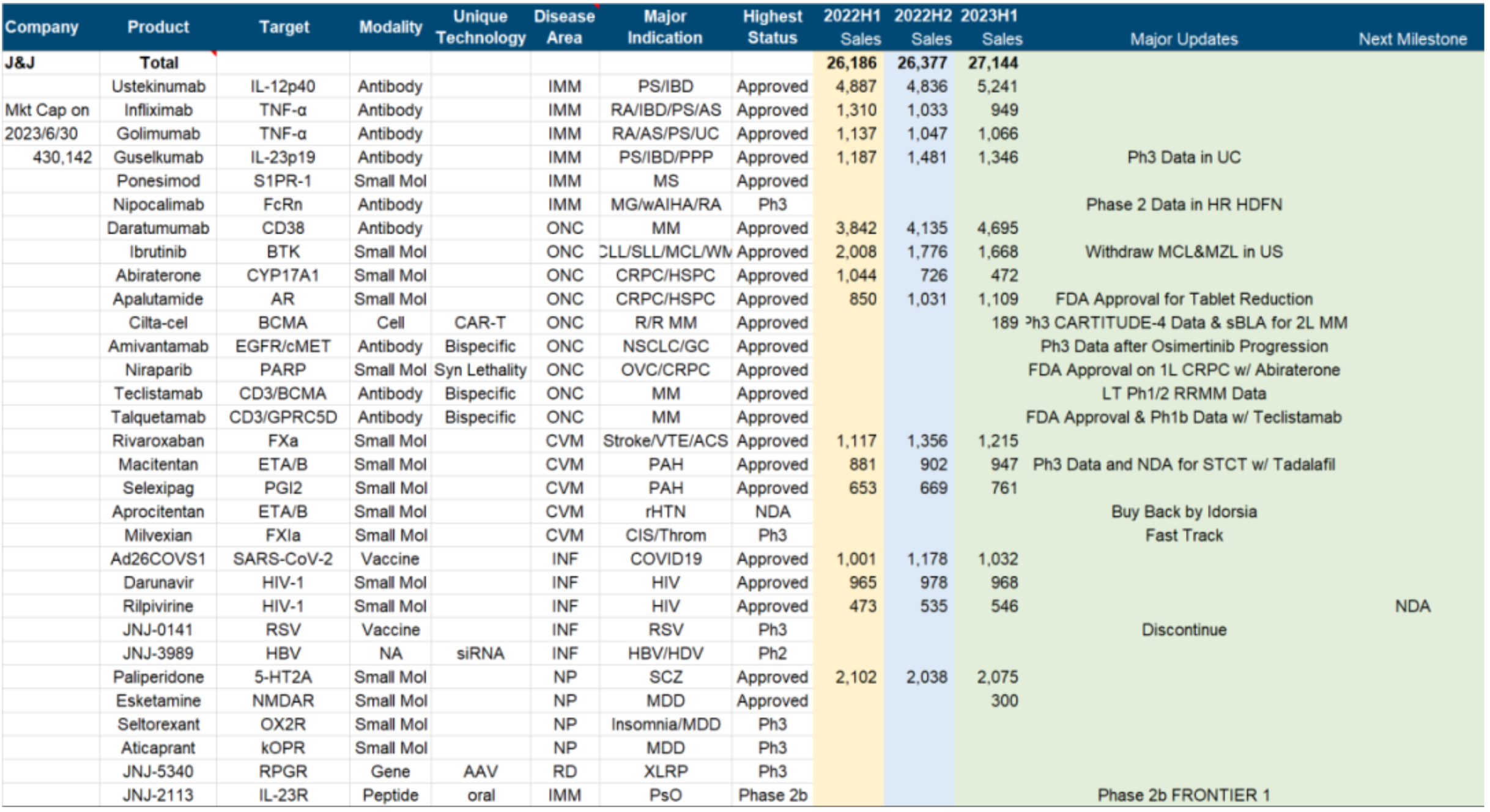
Other noteworthy recent advances include IL23p19 monoclonal antibody Guselkumab announcing phase III clinical data for the treatment of UC, EGFR/cMET bispecific antibody Amivantamab announcing the first phase III clinical data after Osimertinib's progress, the new generation anticoagulant Milvexian receiving Fast Track certification, and oral IL-23R antagonist JNJ-2113 announcing clinical 2b data for the treatment of plaque psoriasis. On the downside, Ibrutinib has withdrawn its MCL and MZL indications, possibly leaving room for AZ and BeiGene to attack. The RSV vaccine was ruthlessly abandoned in phase III, possibly due to feeling powerless against GSK/Pfizer/Moderna, and Aprocitentan was sold back to partner Idorsia after having already submitted its NDA.