Latest Developments in the Research and Development of CB1 agonists
CB1 receptors are a crucial component of the endocannabinoid system found in the human body. These receptors are primarily located in the central nervous system, particularly in the brain. CB1 receptors play a vital role in regulating various physiological processes such as pain perception, appetite, mood, memory, and motor coordination. Activation of CB1 receptors by endocannabinoids or external cannabinoids like THC (tetrahydrocannabinol) can have profound effects on these functions. Understanding the role of CB1 receptors is essential for developing therapeutic interventions targeting the endocannabinoid system, which holds promise in treating various conditions including pain, neurological disorders, and mental health issues.
CB1 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 106 CB1 drugs worldwide, from 105 organizations, covering 110 indications, and conducting 412 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.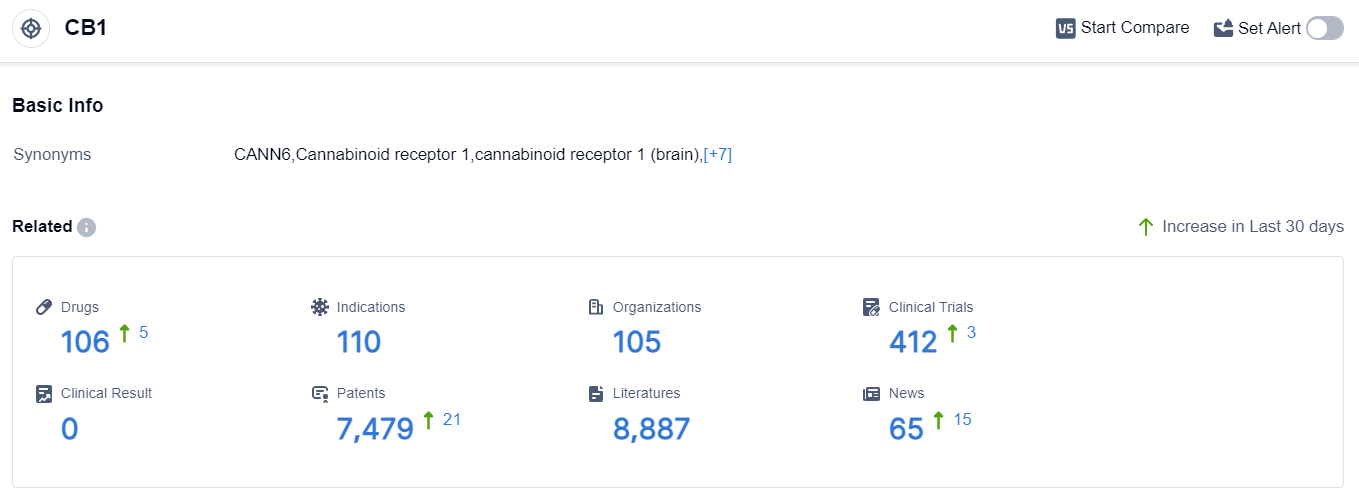
Based on the analysis of the provided data, the current competitive landscape of target CB1 in the pharmaceutical industry is characterized by the involvement of multiple companies at different stages of development. Sanofi is the leading company with drugs approved and in Phase 3.
The indications for drugs under target CB1 are diverse, including obesity, neuralgia, cancer pain, and various other conditions. Small molecule drugs are the most rapidly progressing drug type, followed by monoclonal antibodies, diagnostic radiopharmaceuticals, unknown drugs, and chemical drugs.
The development of target CB1 is happening globally, with countries like the European Union, Canada, Germany, Mexico, and others actively involved. Further analysis is required to assess the future development and potential of target CB1 in the pharmaceutical industry.
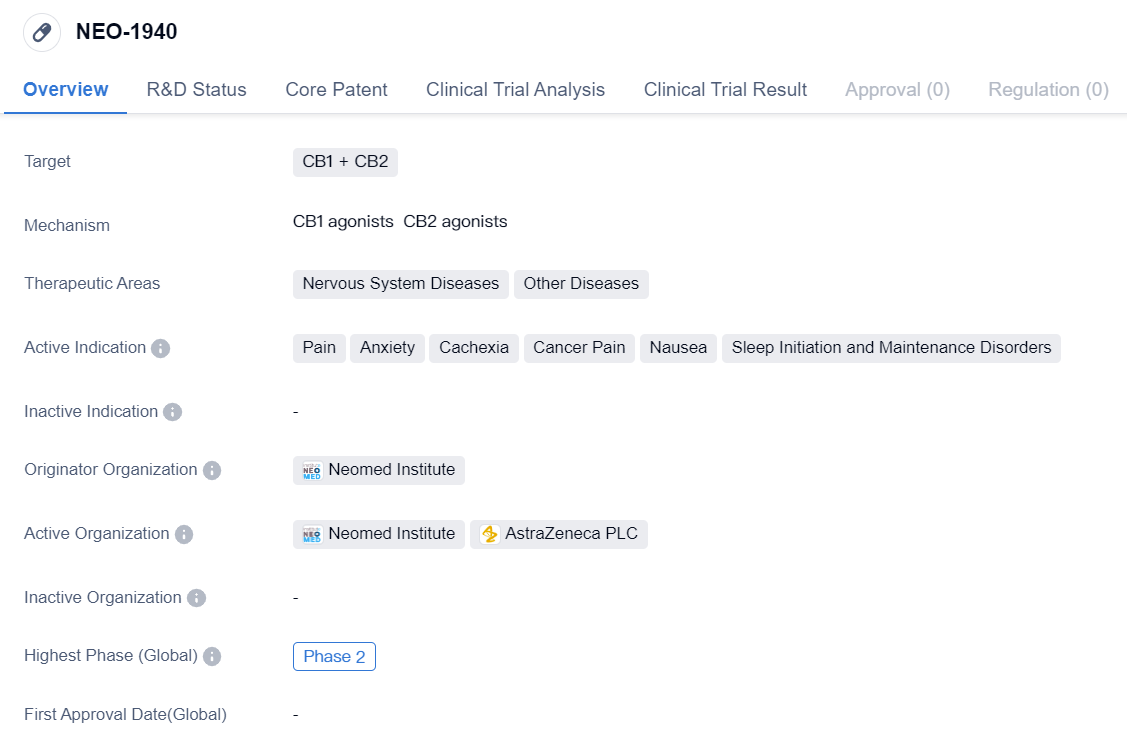
CB1 Agonists that have entered Phase II Clinical Trials: NEO-1940
NEO-1940 is a small molecule drug that targets CB1 and CB2 receptors. It is being developed by Neomed Institute and is currently in Phase 2. The drug has shown potential therapeutic applications in various areas, particularly in the treatment of nervous system diseases and other diseases. It has been specifically indicated for the management of pain, anxiety, cachexia, cancer pain, nausea, and sleep initiation and maintenance disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The targeting of CB1 and CB2 receptors suggests that NEO-1940 may have an impact on the endocannabinoid system, which plays a crucial role in regulating various physiological processes. CB1 receptors are primarily found in the central nervous system, while CB2 receptors are predominantly located in the immune system. By targeting both receptors, NEO-1940 may have the potential to modulate pain perception, reduce anxiety, and alleviate other symptoms associated with the therapeutic areas mentioned.
Neomed Institute, the originator organization of NEO-1940, is likely to be responsible for the research, development, and clinical trials of the drug. As it has reached Phase 2, it indicates that NEO-1940 has shown promising results in preclinical studies and early-stage clinical trials. Phase 2 trials typically involve a larger number of participants and aim to further evaluate the drug's safety and efficacy.
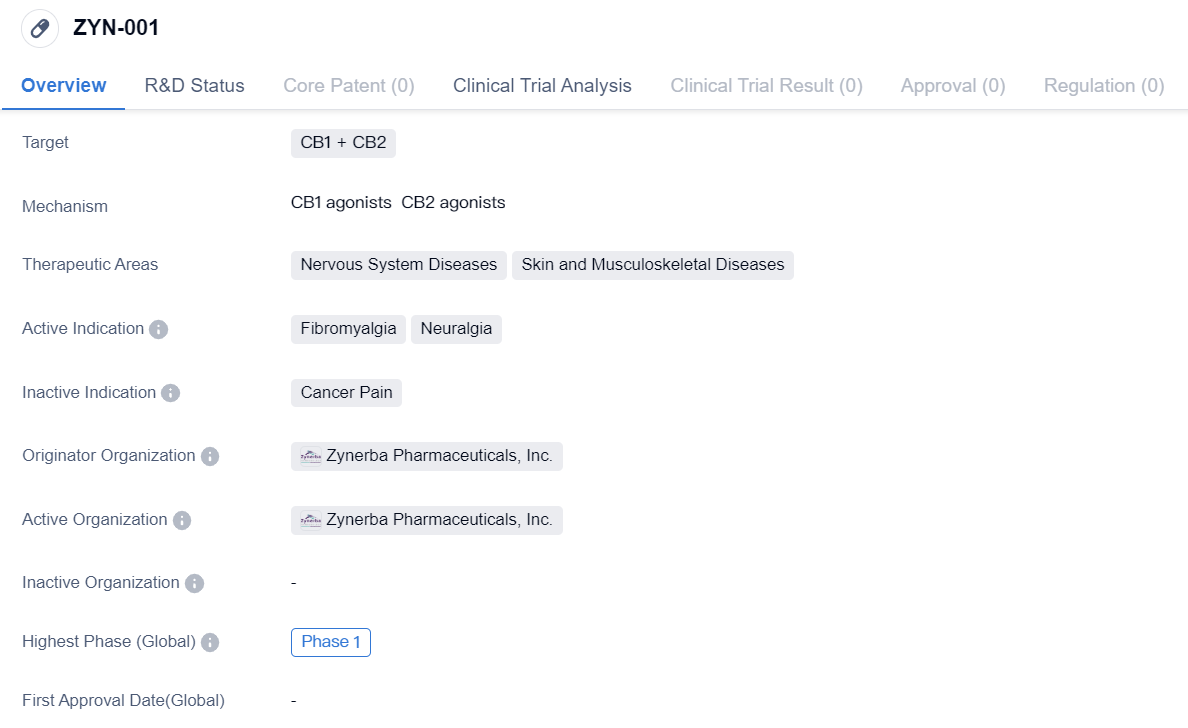
CB1 Agonists that have entered Phase I Clinical Trials:ZYN-001
ZYN-001 is a small molecule drug developed by Zynerba Pharmaceuticals, Inc. It is designed to target CB1 and CB2 receptors in the body. The drug falls under the therapeutic areas of Nervous System Diseases and Skin and Musculoskeletal Diseases.
The active indications for ZYN-001 are Fibromyalgia and Neuralgia, both of which are conditions related to the nervous system. Fibromyalgia is a chronic disorder characterized by widespread musculoskeletal pain, fatigue, and tenderness in localized areas. Neuralgia, on the other hand, refers to severe, shooting pain that occurs along the path of a nerve.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Zynerba Pharmaceuticals, Inc. is the originator organization behind ZYN-001. As of the latest available information, the drug has reached Phase 1 of clinical development. Phase 1 is typically the initial stage of clinical trials where the drug's safety, dosage, and potential side effects are evaluated in a small group of healthy volunteers.
Based on the provided information, ZYN-001 shows promise in the treatment of Fibromyalgia and Neuralgia. By targeting CB1 and CB2 receptors, the drug may have the potential to modulate pain and provide relief to patients suffering from these conditions. However, it is important to note that the information provided does not indicate the current status of the drug's development or its efficacy and safety profile.
Further research and clinical trials will be necessary to determine the effectiveness and safety of ZYN-001 in treating Fibromyalgia and Neuralgia. The drug's progression through subsequent phases of clinical development will provide more insights into its potential as a therapeutic option for patients with these conditions.