Latest Developments in the Research and Development of CGAS Inhibitors
CGAS (Cyclic GMP-AMP Synthase) is a crucial enzyme in the human body that plays a vital role in the innate immune response. It acts as a sensor for detecting foreign DNA, such as viral or bacterial DNA, within the cytoplasm of cells. Upon recognition, CGAS catalyzes the synthesis of cyclic GMP-AMP (cGAMP), which serves as a secondary messenger to activate the STING pathway. This activation triggers the production of type I interferons and pro-inflammatory cytokines, leading to the initiation of an immune response against the invading pathogens. Understanding the role of CGAS provides valuable insights into the mechanisms of immune defense and aids in the development of novel therapeutic strategies.
CGAS Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 10 Sep 2023, there are a total of 6 CGAS drugs worldwide, from 8 organizations, covering 9 indications, and conducting 0 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.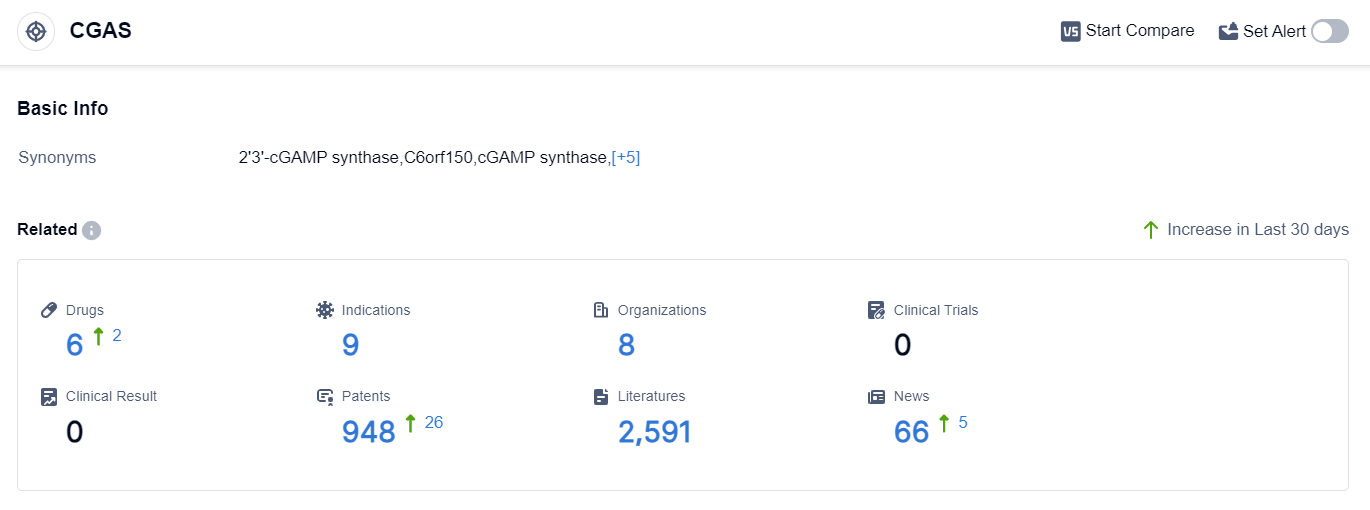
Based on the analysis of the provided data, Ventus Therapeutics, Inc. is the company with the highest development phase under the target CGAS, followed by several other companies in the preclinical phase.
Inflammation is the most common indication being targeted, with other indications also being explored. Small molecule drugs are progressing rapidly, while chemical drugs are in the inactive phase.
The United States is leading in the development of drugs under the target CGAS, with China also showing progress. Overall, the current competitive landscape suggests a diverse range of companies and drug types involved in the development of drugs targeting CGAS, with a focus on inflammation and other related indications. Further research and development efforts are needed to advance these drugs to higher phases and ultimately bring them to market.
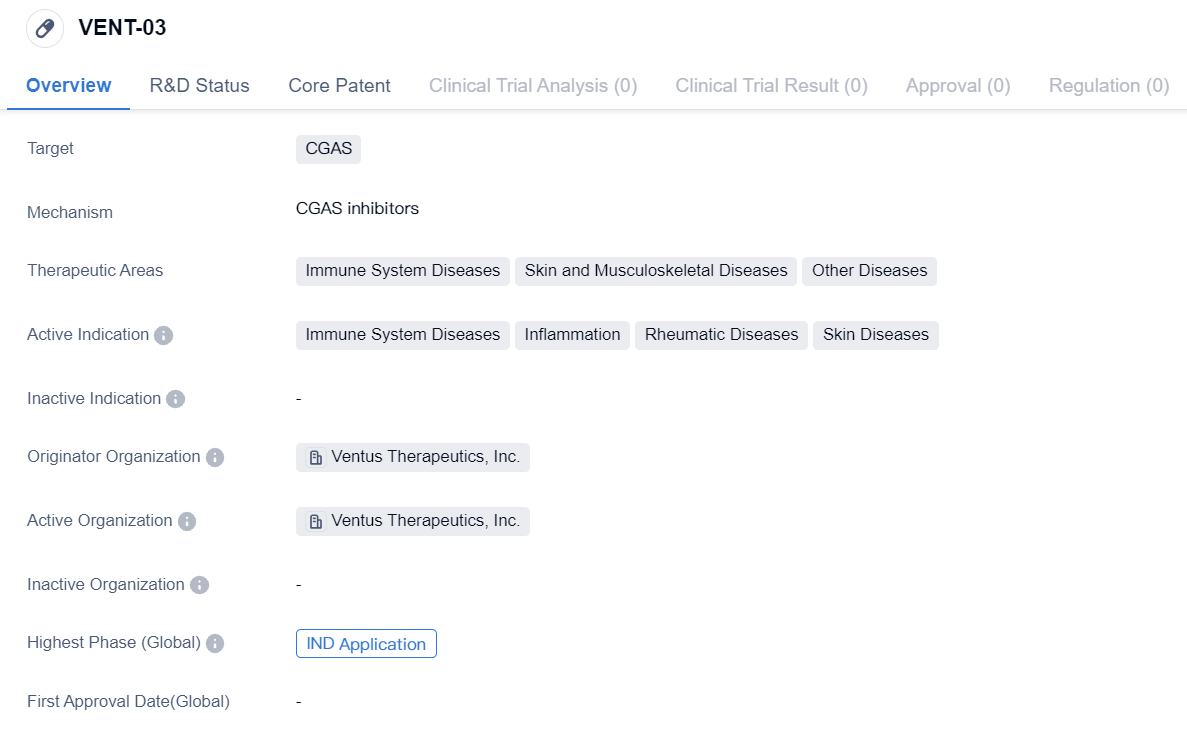
CGAS inhibitor at the IND Application stage: VENT-03
VENT-03 is a small molecule drug developed by Ventus Therapeutics, Inc. It is designed to target CGAS, a protein involved in immune system regulation. The drug is primarily intended for the treatment of immune system diseases, skin and musculoskeletal diseases, and other diseases.
The active indications of VENT-03 include immune system diseases, inflammation, rheumatic diseases, and skin diseases. This suggests that the drug may have a broad therapeutic potential in addressing various conditions related to the immune system and inflammatory processes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, VENT-03 is likely to have a relatively small molecular size, allowing it to easily penetrate cell membranes and interact with its target, CGAS. Small molecule drugs are commonly used in pharmaceutical development due to their favorable pharmacokinetic properties and ease of formulation.
Ventus Therapeutics, Inc. is the originator organization behind VENT-03. As the developer of the drug, Ventus Therapeutics is responsible for conducting preclinical and clinical studies to evaluate its safety and efficacy. The fact that VENT-03 has reached IND application indicates that it has undergone significant preclinical testing and is now ready for evaluation in human clinical trials.
The therapeutic areas targeted by VENT-03, including immune system diseases, skin and musculoskeletal diseases, and other diseases, highlight the potential versatility of the drug. Immune system diseases encompass a wide range of conditions, such as autoimmune disorders and immunodeficiencies. Skin and musculoskeletal diseases may include conditions like psoriasis, rheumatoid arthritis, and osteoarthritis. The inclusion of "other diseases" suggests that VENT-03 may have additional applications beyond the specified therapeutic areas.
In summary, VENT-03 is a small molecule drug developed by Ventus Therapeutics, Inc. It targets CGAS and shows potential for treating immune system diseases, inflammation, rheumatic diseases, and skin diseases. As the drug has reached IND application, it is poised to enter human clinical trials, where its safety and efficacy will be further evaluated.
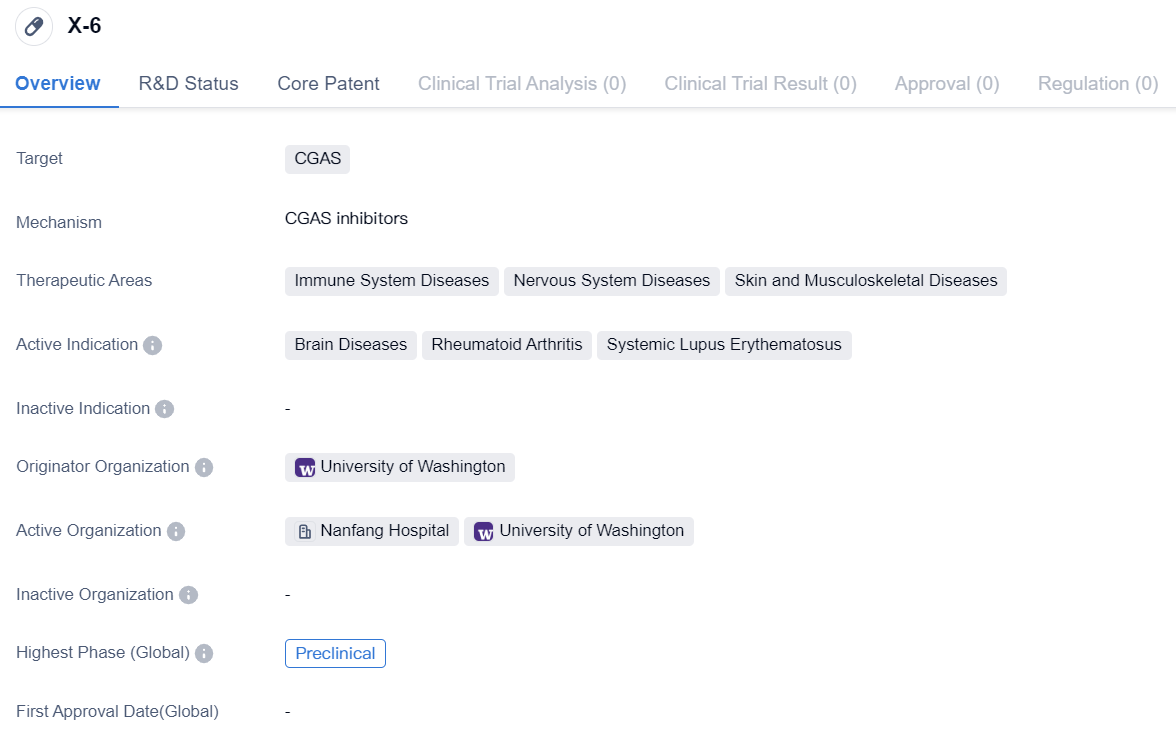
CGAS inhibitor in the preclinical research stage: X-6
X-6 is a small molecule drug that targets CGAS. It is being developed for the treatment of immune system diseases, nervous system diseases, and skin and musculoskeletal diseases. The active indications for this drug include brain diseases, rheumatoid arthritis, and systemic lupus erythematosus.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of X-6 is the University of Washington. Currently, the drug is in the preclinical phase, which means it is still undergoing laboratory testing and has not yet been tested on humans. This is the highest phase of development for X-6 both globally and in China.
As a small molecule drug, X-6 is likely to have a low molecular weight and can easily penetrate cell membranes. This makes it a promising candidate for the treatment of various diseases. By targeting CGAS, X-6 aims to modulate the immune response and potentially alleviate symptoms associated with immune system diseases.
The therapeutic areas of X-6 indicate its potential to address a wide range of diseases affecting the immune system, nervous system, and skin and musculoskeletal systems. Brain diseases, such as neurodegenerative disorders or neurological conditions, could benefit from the targeted action of X-6. Rheumatoid arthritis, an autoimmune disease causing joint inflammation, and systemic lupus erythematosus, a chronic autoimmune condition affecting multiple organs, are also active indications for X-6.
Being in the preclinical phase, X-6 is still in the early stages of development. Further research and testing will be required to determine its safety and efficacy in humans. If successful, X-6 could potentially offer new treatment options for patients suffering from immune system diseases, nervous system diseases, and skin and musculoskeletal diseases.
In conclusion, X-6 is a small molecule drug targeting CGAS, with potential applications in immune system diseases, nervous system diseases, and skin and musculoskeletal diseases. It is currently in the preclinical phase of development, and its active indications include brain diseases, rheumatoid arthritis, and systemic lupus erythematosus. The University of Washington is the originator organization of this promising drug.