LIB Therapeutics Reveals Positive Phase 3 Lerodalcibep Data at EAS Congress
LIB Therapeutics Inc., a private, late-stage biopharma firm, is developing Lerodalcibep, a novel, monthly, LDL-cholesterol reducing, third-generation PCSK9 inhibitor. The company announced favorable outcomes from two investigations in the final Phase 3 LIBerate registration-enabling program. These findings were presented at the 92nd European Atherosclerosis Society Congress held in Lyon, France, from May 26-29, 2024.
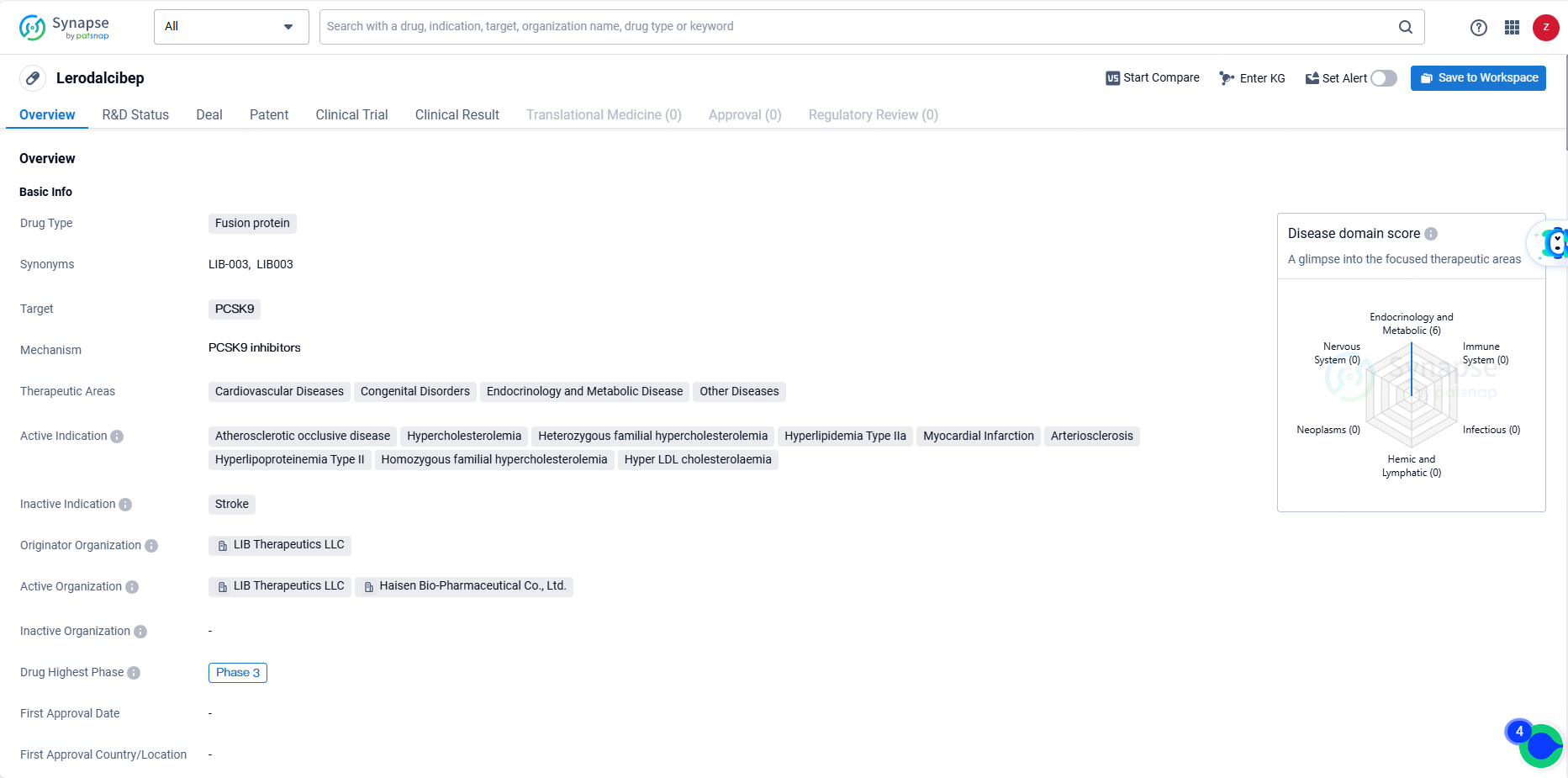
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The international Phase 3 LIBerate-CVD study, involving 922 participants, was a randomized, double-blind, placebo-controlled trial aimed at assessing the 52-week efficiency and safety of Lerodalcibep in patients with either a history of cardiovascular disease (CVD) or those at very high risk for CVD, while on stable lipid-lowering treatment. The primary efficacy endpoints included the percentage change in LDL-C levels from the baseline at Week 52 and the average at Weeks 50 and 52. Secondary metrics comprised the achievement of EAS/ESC LDL-C targets, as well as other lipid and apolipoprotein alterations.
Participants were divided in a 2:1 ratio to receive either a 300 mg single monthly subcutaneous dose of Lerodalcibep or a placebo for 52 weeks. Around 85% of the participants had CVD, with approximately 30% being female. The mean baseline LDL-C level was recorded at 102 mg/dL, despite 85% of the patients being on statins and 15% on ezetimibe.
“LIBerate-CVD represents the pivotal registration-enabling trial in our global Phase 3 LIBerate program for Lerodalcibep,” stated Evan Stein, MD, PhD, Chief Scientific Officer at LIB Therapeutics. “Our LIBerate initiative included a diverse range of global participants with CVD, including those at very high and high risk, as well as individuals with heterozygous and homozygous familial hypercholesterolemia (FH), aiming to support a comprehensive label upon approval. The success of these international trials is attributed to the unwavering commitment and enthusiasm of investigators and patients worldwide.”
“These findings on Lerodalcibep further corroborate its potential as a top-tier candidate with significant, enduring LDL-C lowering effects through a patient-convenient, once-monthly, single subcutaneous injection that remains stable at room temperature, eliminating the need for refrigeration at home or during travel,” commented David Cory, CEO of LIB Therapeutics. “We anticipate submitting a Biologics License Application to the FDA later this year, to be followed by a Marketing Authorization Application to the European Medicines Agency.”
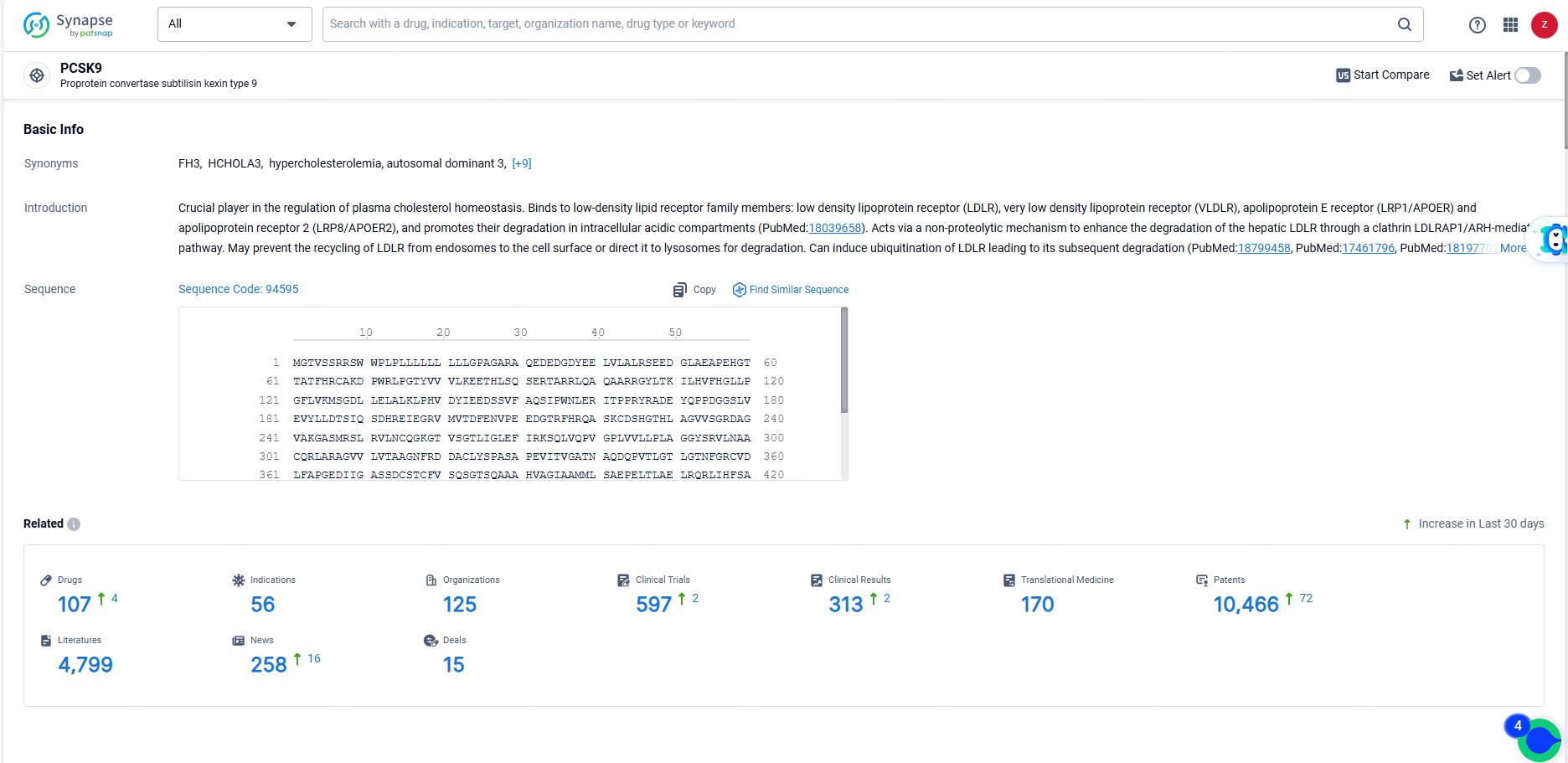
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 3, 2024, there are 107 investigational drugs for the PCSK9 target, including 56 indications, 125 R&D institutions involved, with related clinical trials reaching 597, and as many as 10466 patents.
Lerodalcibep is a novel, potent, small binding protein, third-generation PCSK9 inhibitor, and has been developed as a more convenient, once-monthly, single subcutaneous injection with long-ambient stability. In clinical trials, Lerodalcibep has demonstrated sustained LDL-C reductions and is expected to expand treatment options for the millions of patients around the world with atherosclerotic cardiovascular disease and those at very high and high risk for ASCVD, including the 30 million individuals with more severe inherited high-cholesterol called familial hypercholesterolemia.