March 2023 Global Innovative Drug Report
The recently published Global Innovative Drug Report, based on data from the PatSnap Synapse database, consists of three parts:
1.Global Approved Drugs
2.Global Drugs under Expedited Review Pathway
3.Global Drug Clinical Progression
The report offers a comprehensive overview of the latest advancements in the pharmaceutical industry.
Global Innovative Drug Report Overview
1.) March 2023 Global Drug Approvals:
During March of 2023, a total of 41 drugs were approved on a global scale, encompassing a diverse range of drug types. These include 21 Small Molecule drugs, 4 Vaccines, 3 Antibody drugs, 3 Synthetic Peptides, 2 Enzymes, 2 Biosimilars, 1 Antibody Drug Conjugate (ADC), 1 Bispecific Antibody, 1 Fusion Protein, 1 Immunoglobulin, 1 Cell Therapy, and 1 Fc Fragment.
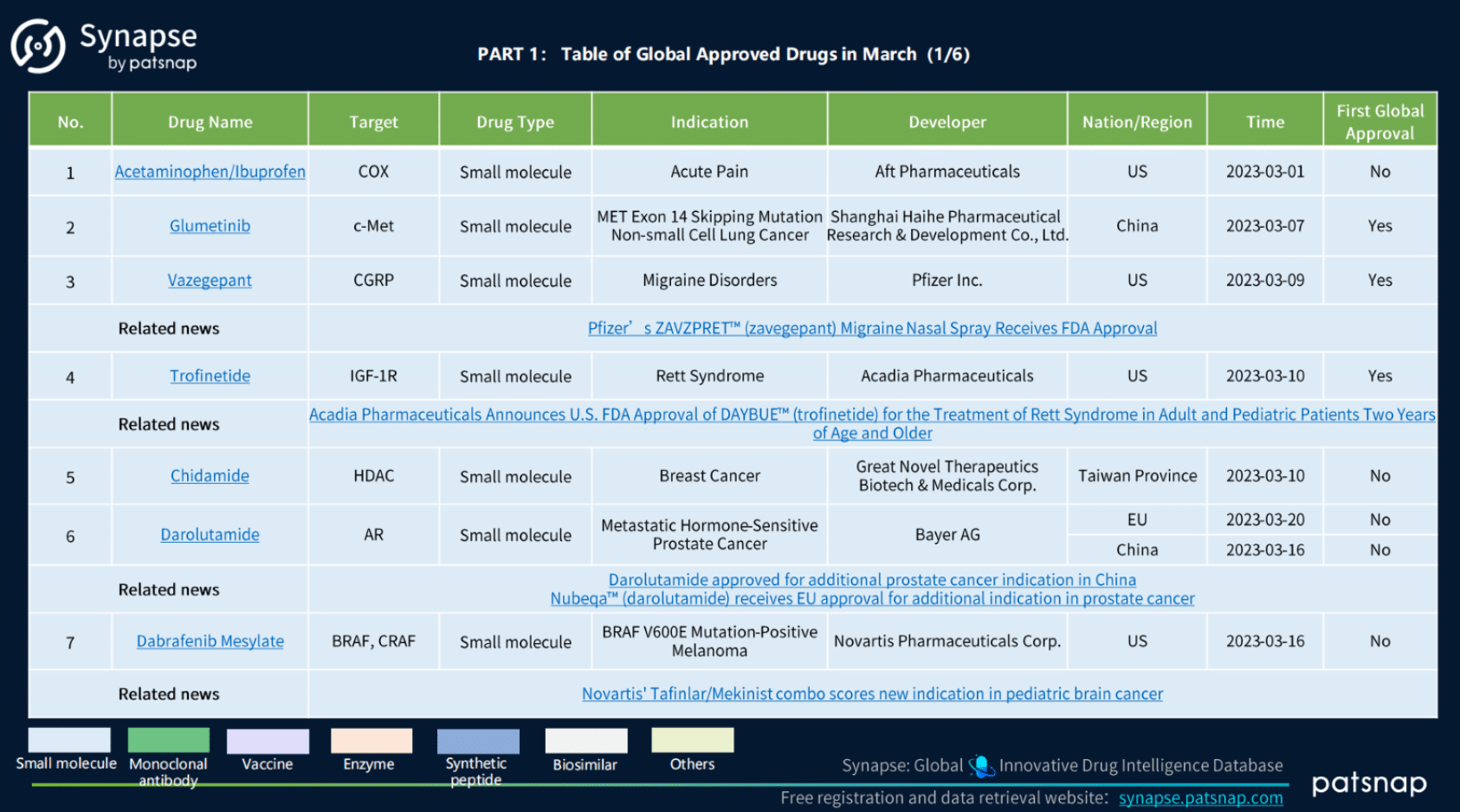
March 2023 Global Approved Drugs,Synapse
2.) March 2023 Global Drugs Subject to Expedited Review:
During March 2023, the global pharmaceutical industry had a total of 53 drugs undergoing expedited review pathway, comprising various types of designations. Notably, 32 drugs had received Orphan Drug Designation, while 6 were designated as Fast Track, and 4 were granted Breakthrough Therapy Designation. Additionally, 4 drugs obtained Conditional Marketing Approval, 3 received Emergency Use Authorization, 1 underwent Priority Review, 1 was granted PRIME designation, 1 received Rare Pediatric Disease designation, and 1 achieved Accelerated Approval.
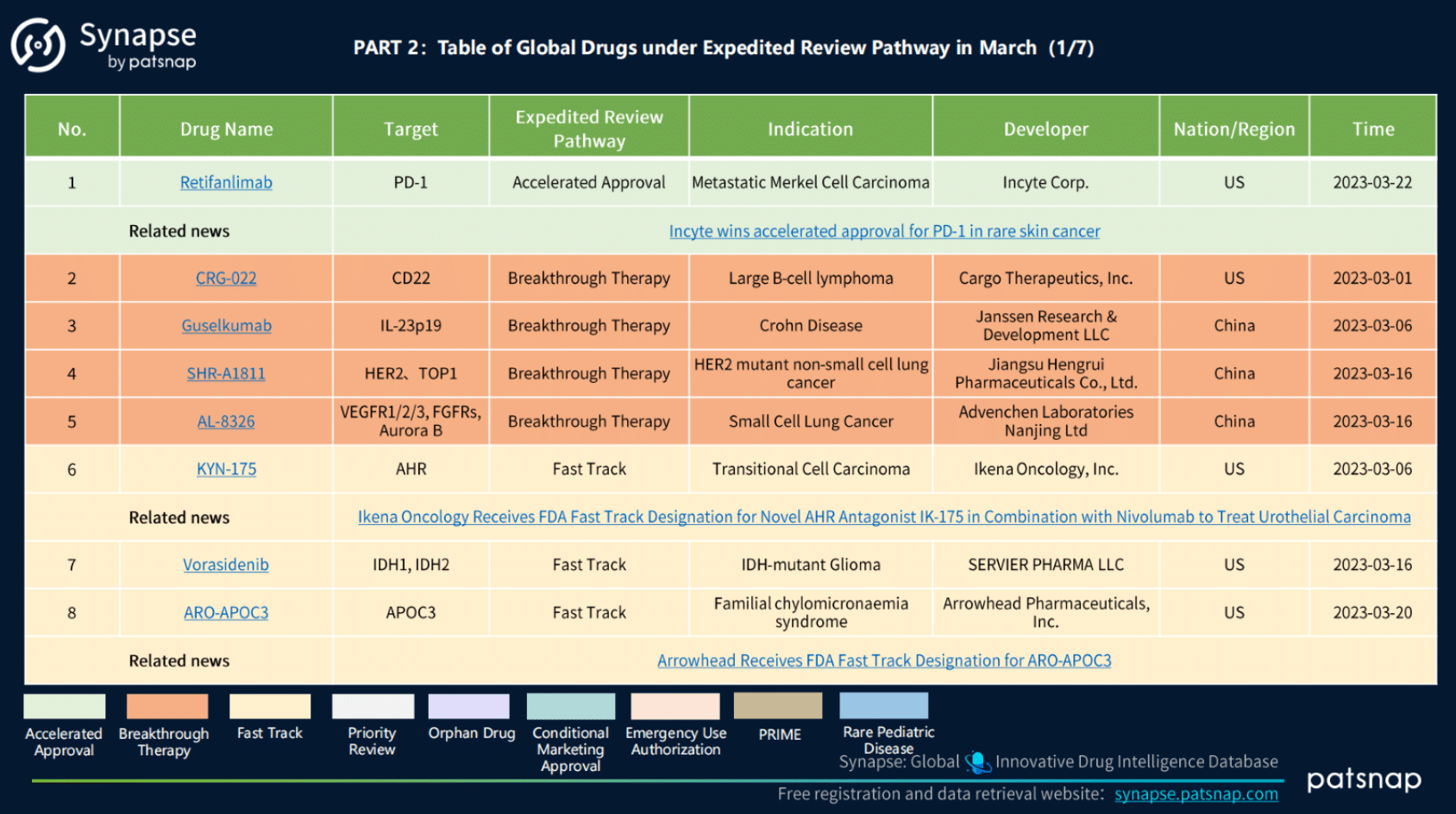
March 2023 Global Drugs Subject to Expedited Review, Synapse
3.) March 2023 Global Clinical Progress of Drugs:
During March 2023, a total of 3352 news items related to global drug clinical progress were available for retrieval from the Synapse database. From this comprehensive collection, 10 noteworthy news items were handpicked, including Clinical Reviews and Approvals, Phase I, II, and III Results, as well as Marketing Authorization Applications. These 10 selected news items represent the most significant and influential developments in the pharmaceutical industry during the month of March 2023.
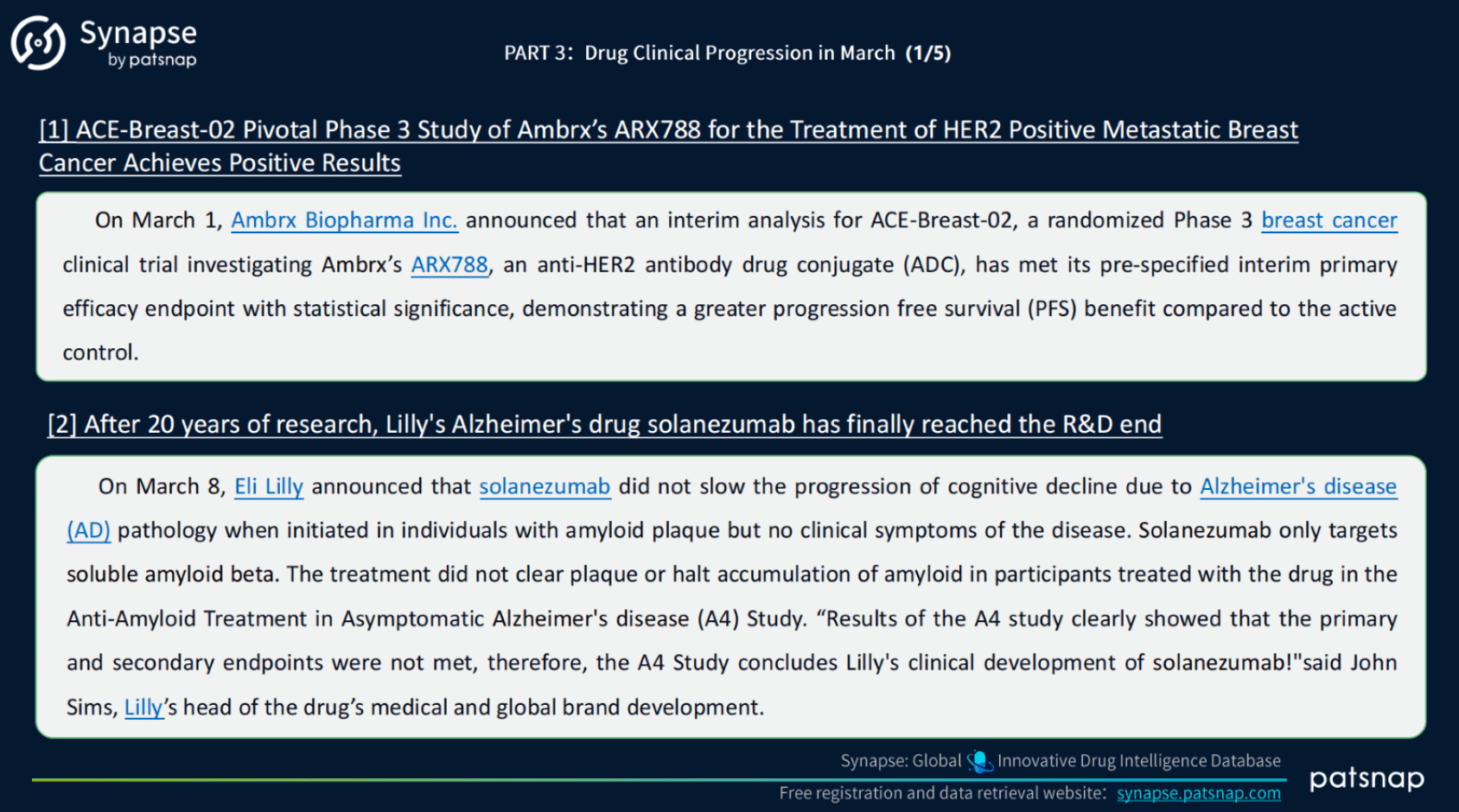
March 2023 Global Clinical Progress of Drugs, Synapse
Gain Access to the Comprehensive Report for FREE – Download Now! If you aren’t registered for Synapse (registration is required to download the report), click here to register for free.

Copyright Statement: This report is the sole property of PatSnap and is protected under copyright laws. Any reproduction, excerpting, or other use of this report without explicit authorization from PatSnap is strictly prohibited. Authorized products must be used within the scope of authorization and must include a clear indication of the source. PatSnap reserves the right to investigate any violations of this statement and pursue legal action as necessary. For inquiries regarding authorization, please contact phs@patsnap.com.

