May 2025 Patent Highlights: Eli Lilly’s CRHR2 peptide agonists
Lilly is emerging as a new a new leade in the treatment of obesity and diabetes , particularly with its recent glucose-dependent insulinotropic polypeptide (GIP)/glucagon-like peptide-1 (GLP-1) receptor agonist, tirzepatide, which met the primary endpoint and all five key secondary endpoints in the SURMOUNT-5 trial, demonstrating superior efficacy compared to semaglutide, further solidifying its position as a frontrunner.
Lilly’s success may be exerting pressure on its competitors. In May, Novo Nordisk announced that Lars Fruergaard Jørgensen will step down from his role as CEO of Novo Nordisk. The company stated that this decision reflects recent market challenges, a decline in stock price, and the intentions of the Novo Nordisk Foundation. The board and Jørgensen mutually agreed that initiating a CEO succession process is in the best interest of the company and its shareholders.
Tirzepatide is not Lilly’s only contender in this field; its small-molecule agonist orforglipron also met primary endpoints in studies and is poised for regulatory submission and potential approval. Now, with the disclosure of patent WO2025106761A1, Lilly has revealed new developments in this field.
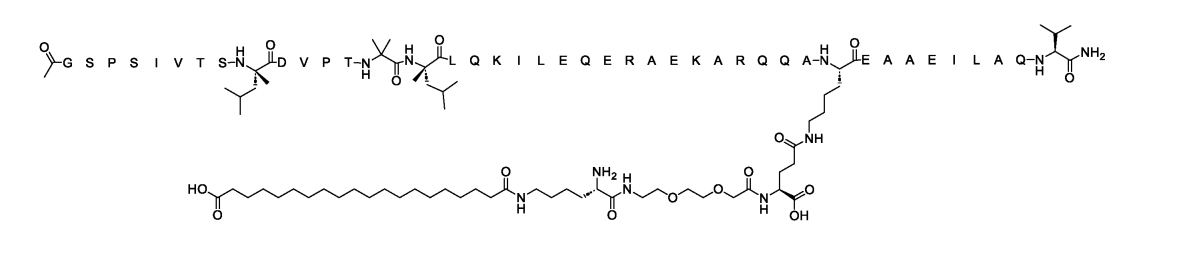
SEQ ID NO:1(WO2025106761A1)
hCRHR2 cAMP EC50(pM, ± SE):9.3±1.1
A7r5 cAMP EC50(pM, ± SE):157±8
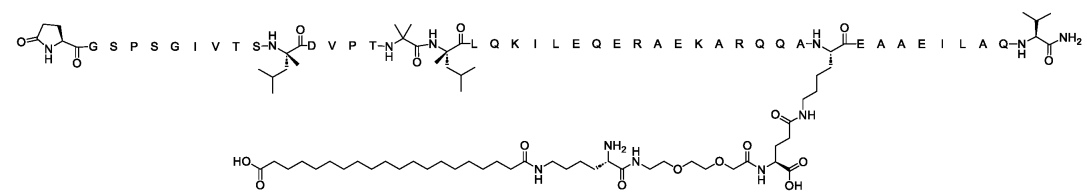
SEQ ID NO:22(WO2025106761A1)
hCRHR2 cAMP EC50(pM, ± SE):11.3±3.0
A7r5 cAMP EC50(pM, ± SE):314±24
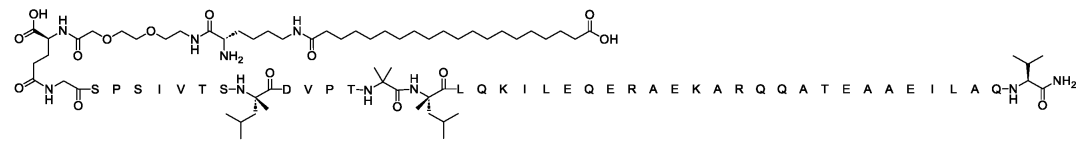
SEQ ID NO:25(WO2025106761A1)
hCRHR2 cAMP EC50(pM, ± SE):6.9±0.5
A7r5 cAMP EC50(pM, ± SE):112±5
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!