Maze Therapeutics Commences First-stage Trial with MZE829 Oral Drug, Targeting APOL1-mediated Renal Conditions
Maze Therapeutics, a firm dedicated to converting genetic discoveries into innovative targeted therapies, has commenced the administration phase of its Phase 1 clinical evaluation of MZE829 to test subjects in good health. MZE829 is a small molecule inhibitor taken orally and specifically targets the apolipoprotein L1 (APOL1) protein. It aims to impede the APOL1 channel activity and mitigate the clinical symptoms associated with APOL1-related renal conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Launching this pivotal study represents the second initiative propelled from our Compass™ technology into the realm of clinical trials, showcasing Maze's capability in utilizing our extensive genetic and clinical data resources to innovate new therapeutic solutions for patients," announced Harold Bernstein, M.D., Ph.D., Maze's lead for research and development as well as the chief medical officer.
Dr. Bernstein further noted, "This trial is crucial for our mission to develop transformative therapies for individuals with prevalent conditions, such as kidney disease, by harnessing our genetics-based approach to pharmaceutical discovery. Our aim is that the insights gained from this initial trial will pave the way for enlarging the scope of MZE829 assessments to include a wider chronic kidney disease demographic affected by APOL1 kidney disease."
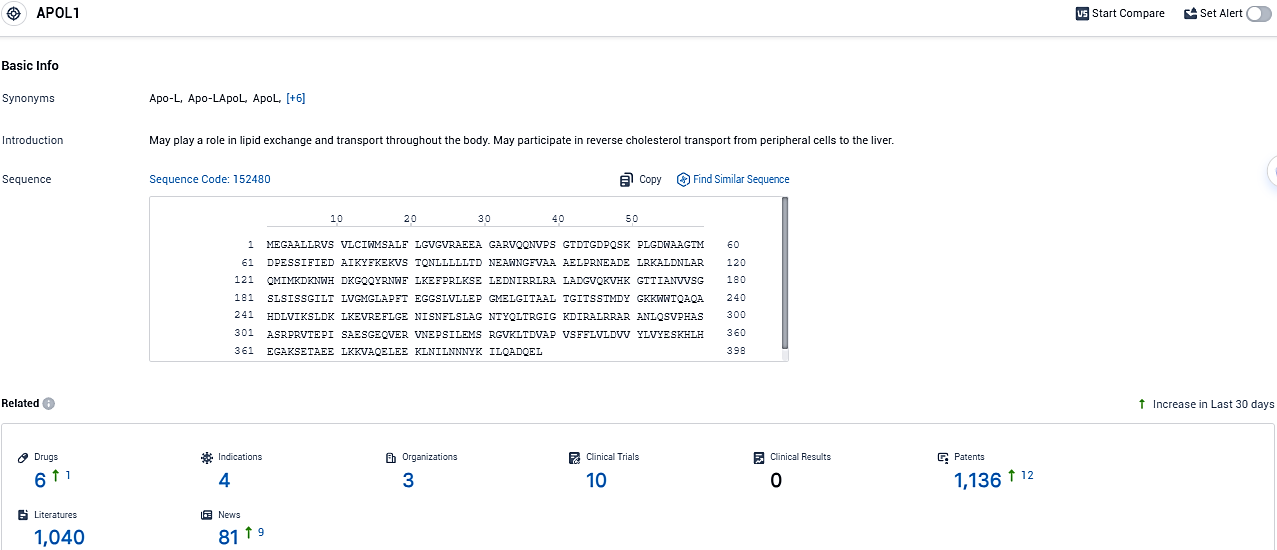
The APOL1 protein, encoded by its eponymous gene in humans, plays a role in kidney health, with certain gene variants linked to an elevated chance of developing a range of kidney disorders, particularly within the African ancestry populations. MZE829 has emerged from data produced using Maze's specialized and innovative platform, Compass™.
Earlier laboratory-based evaluations have evidenced that Maze's APOL1 inhibitor is well-received and has led to a reduction in albuminuria in both chronic and acute models of APOL1 kidney disease crafted to mimic human physiology, further showing improvements in tissue health.
The inaugural Phase 1 trial is a meticulously designed, randomized, and placebo-controlled study assessing single and multiple dose escalations to gauge the safety and pharmacokinetics of MZE829 when taken orally. This study also looks at the effects of food intake on the drug's performance and any possible interactions with other drugs in healthy subjects. Maze expects to release the findings from this investigation in the latter half of 2024, which will inform the progression into a subsequent Phase 2 clinical program involving patients.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
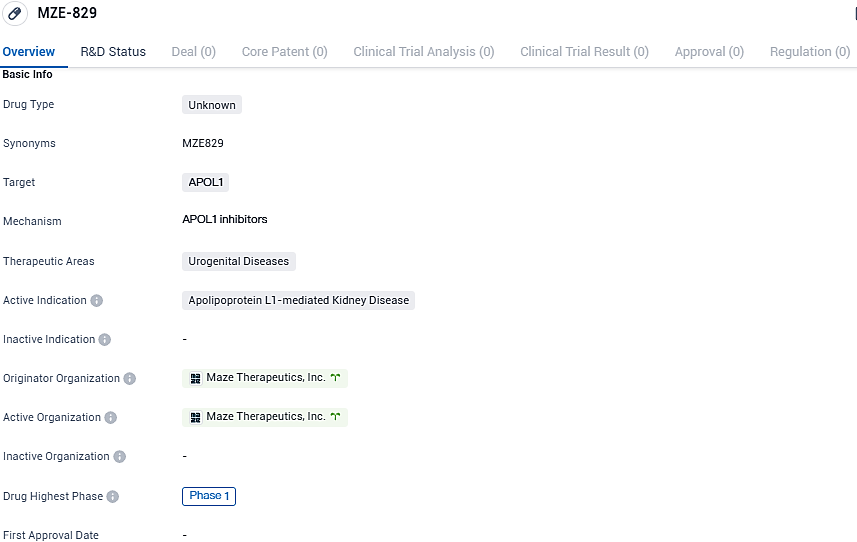
According to the data provided by the Synapse Database, As of December 21, 2023, there are 6 investigational drugs for the APOL1 target, including 4 indications, 3 R&D institutions involved, with related clinical trials reaching 10, and as many as 1136 patents.
MZE-829 targets APOL1 and is intended to treat Apolipoprotein L1-mediated Kidney Disease, a urogenital disease. The drug is currently in Phase 1 of clinical development, indicating that it is undergoing early-stage trials to assess its safety and efficacy.