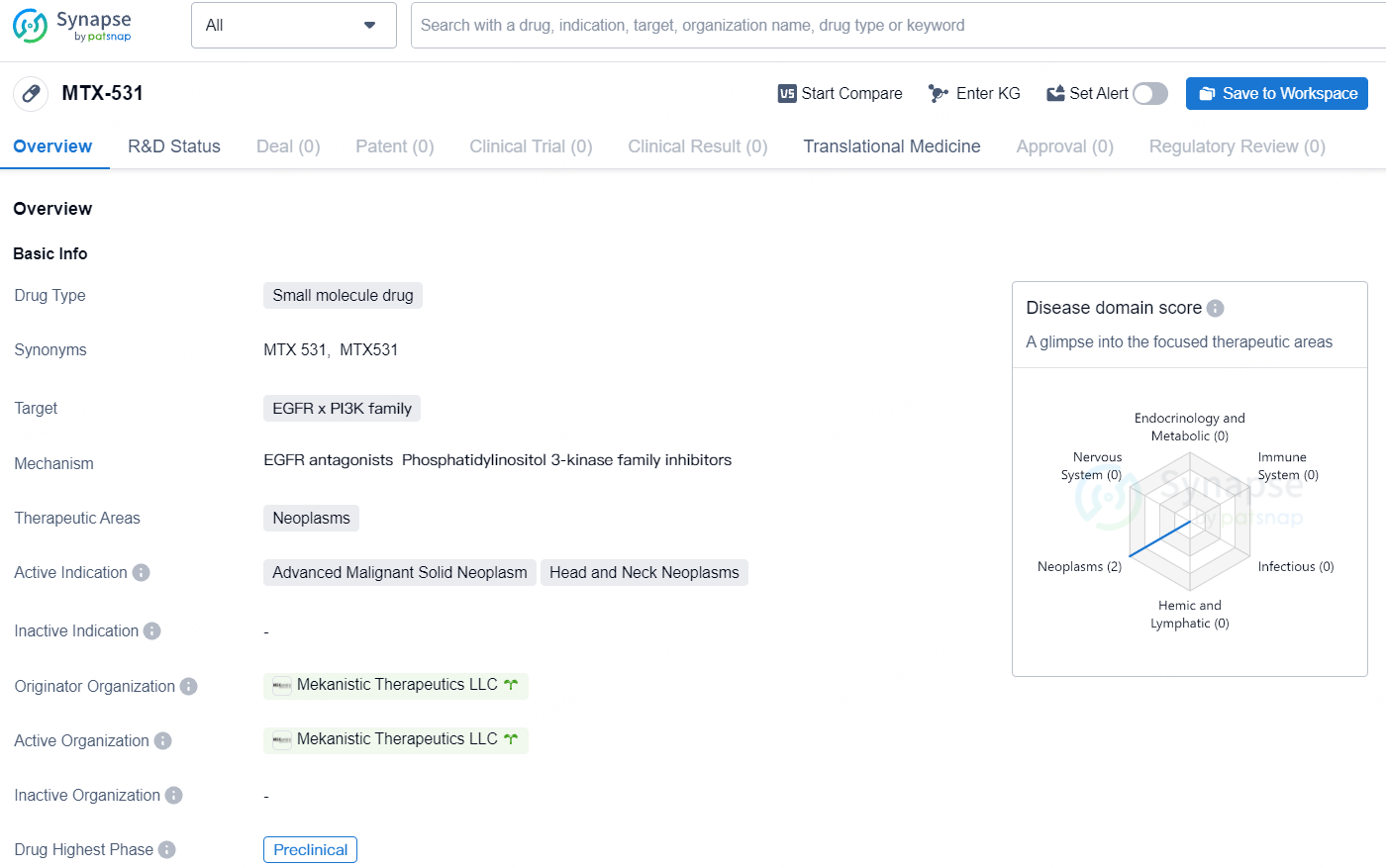
MEKanistic Therapeutics Inc.'s MTX-531 Shows Tolerability and Tumor Reduction in Preclinical Studies
MEKanistic Therapeutics Inc., a biotech firm at the forefront of creating advanced kinase inhibitors for oncology therapies, has disclosed the peer-reviewed publication of recent preclinical studies on its primary candidate, MTX-531, an experimental dual-targeting treatment, in Nature Cancer. MTX-531 is an innovative therapy with the potential to be first-in-class, specifically formulated to inhibit both EGFR and PI3K, two essential proteins associated with cancer cell survival and growth.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We are thrilled to announce the favorable outcomes of MTX-531 preclinical research featured in Nature Cancer. These findings, we believe, indicate the potential of a new approach to tackling the fundamental issue of resistance to current cancer therapies," stated Danny Cunagin, Chief Executive Officer of MEKanistic Therapeutics. "Our dual inhibition strategy targets key adaptive resistance pathways, potentially offering more robust cancer control compared to single-target therapies. We anticipate progressing this groundbreaking therapy into clinical trials for patient treatment."
Preclinical evaluations revealed that MTX-531 is highly potent at nanomolar concentrations against both EGFR and PI3K (14.7 nM for EGFR, 6.44 nM for PI3K), showing strong selectivity via extensive kinome profiling. Furthermore, unlike other pan-PI3K inhibitors, MTX-531 did not induce hyperglycemia in mice at therapeutic doses. This contrasts with other inhibitors known to significantly elevate blood glucose and insulin levels in both preclinical and clinical contexts.
Judith Sebolt-Leopold, PhD, Chief Scientific Officer at MEKanistic Therapeutics, remarked, "MTX-531 stands out as the first PI3K inhibitor that can selectively co-target EGFR and is the first pan-PI3K inhibitor that does not cause hyperglycemia—a common issue leading to the discontinuation of PI3K inhibitor treatments. This distinctive feature, resulting from MTX-531's precise computational design, provides an advantageous therapeutic index and resistance to adaptive resistance mechanisms not seen in previous PI3K inhibitor clinical trials."
In preclinical models of head and neck squamous cell carcinoma (HNSCC), MTX-531 monotherapy led to notable tumor regression. The oral therapy effectively inhibited PI3K and EGFR signaling in a balanced manner, yielding objective responses in all HNSCC models tested. Complete tumor regressions were noted across various dose levels, with survival rates improving from 62% to over 500% across models.
Additionally, in combination with a MEK inhibitor (trametinib) or a KRAS inhibitor (sotorasib), MTX-531 treatment more than doubled the occurrence of tumor regressions, achieving a 100% objective response rate in several KRAS mutant colorectal (CRC) and pancreatic tumor models.
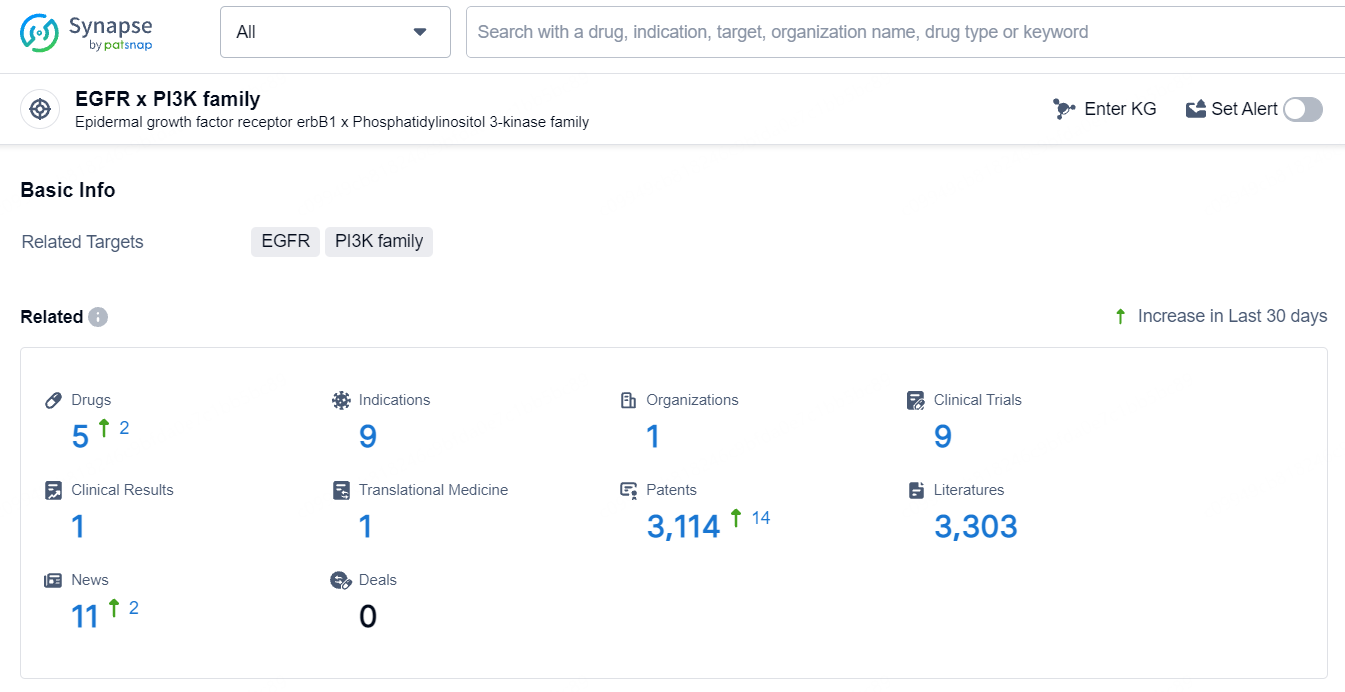
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 16, 2024, there are 5 investigational drugs for the EGFR and PI3K target, including 9 indications, 1 R&D institution involved, with related clinical trials reaching 9, and as many as 3114 patents.
MTX-531 is a small molecule drug with a specific focus on neoplasms, particularly advanced malignant solid neoplasms and head and neck neoplasms. As it progresses through preclinical development, further studies will be needed to evaluate its effectiveness and potential as a therapeutic option for patients with these types of cancers.