Merck Initiates Phase 3 Trial for Bomedemstat in Select Essential Thrombocythemia Cases
Merck (NYSE: MRK), also known as MSD outside of the U.S. and Canada, has announced the launch of Shorespan-007, a critical Phase 3 clinical study focused on assessing the efficacy of bomedemstat. Bomedemstat is an experimental oral lysine-specific demethylase 1 (LSD1) inhibitor being studied for use in treating patients with essential thrombocythemia (ET) who have not previously undergone cytoreductive therapy. Essential thrombocythemia is a chronic and rare blood disorder, and it is the most prevalent subtype of myeloproliferative neoplasm. The Shorespan-007 trial has commenced with patient recruitment happening worldwide.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The care standard for essential thrombocythemia has not changed for decades, and patients are seeking new treatments that can enhance both disease management and quality of life," stated Dr. Gregory Lubiniecki, vice president of global clinical development at Merck Research Laboratories. "We are swiftly progressing our clinical development initiatives with the aim of addressing these unmet needs and providing additional options to those affected by myeloproliferative neoplasms."
Shorespan-007 is a Phase 3, randomized, double-blind, active comparator-controlled clinical trial (NCT06456346) assessing bomedemstat in comparison to the current standard chemotherapy, hydroxyurea, for ET patients who have not previously undergone cytoreductive therapy. Approximately 300 patients worldwide will be recruited for the trial.
The primary outcome measure is the durable clinicohematologic response rate (CHR). Important secondary outcomes include the Myelofibrosis Symptom Assessment Form version 4.0 (MFSAF v4.0) individual fatigue symptom item score, the Patient-reported Outcomes Measurement Information System (PROMIS) Fatigue SF-7a total fatigue score, and the MFSAF v4.0 total symptom score. Other secondary outcomes include the duration of hematologic remission, event-free survival, and the rate of disease progression.
As previously disclosed, bomedemstat is also being explored in Shorespan-006, another Phase 3, global, randomized, open-label, active comparator-controlled trial (NCT06079879), examining bomedemstat against the best available therapy for roughly 300 ET patients who show inadequate response to or intolerance of hydroxyurea.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
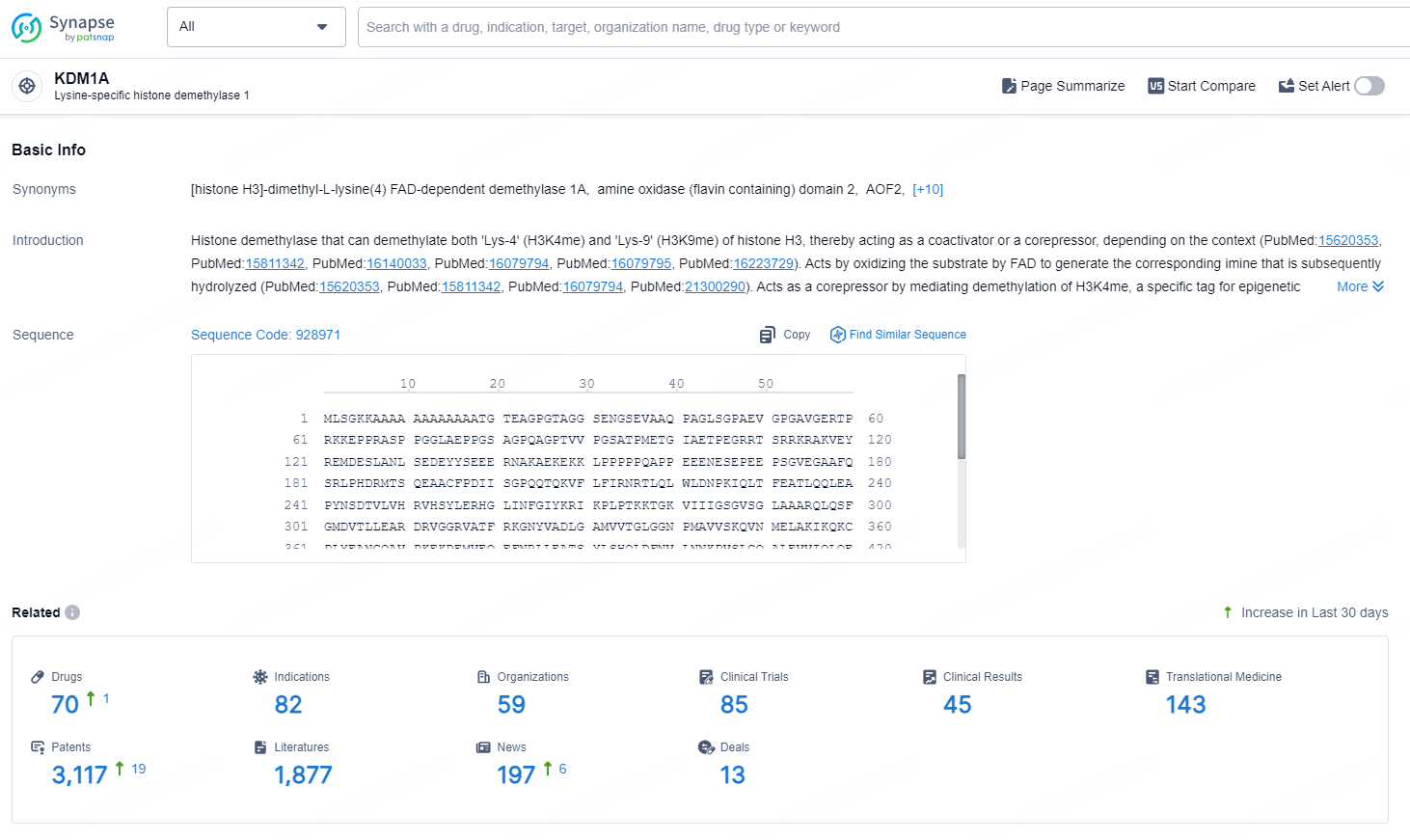
According to the data provided by the Synapse Database, As of August 30, 2024, there are 70 investigational drugs for the KDM1A targets, including 82 indications, 59 R&D institutions involved, with related clinical trials reaching 85, and as many as 3117 patents.
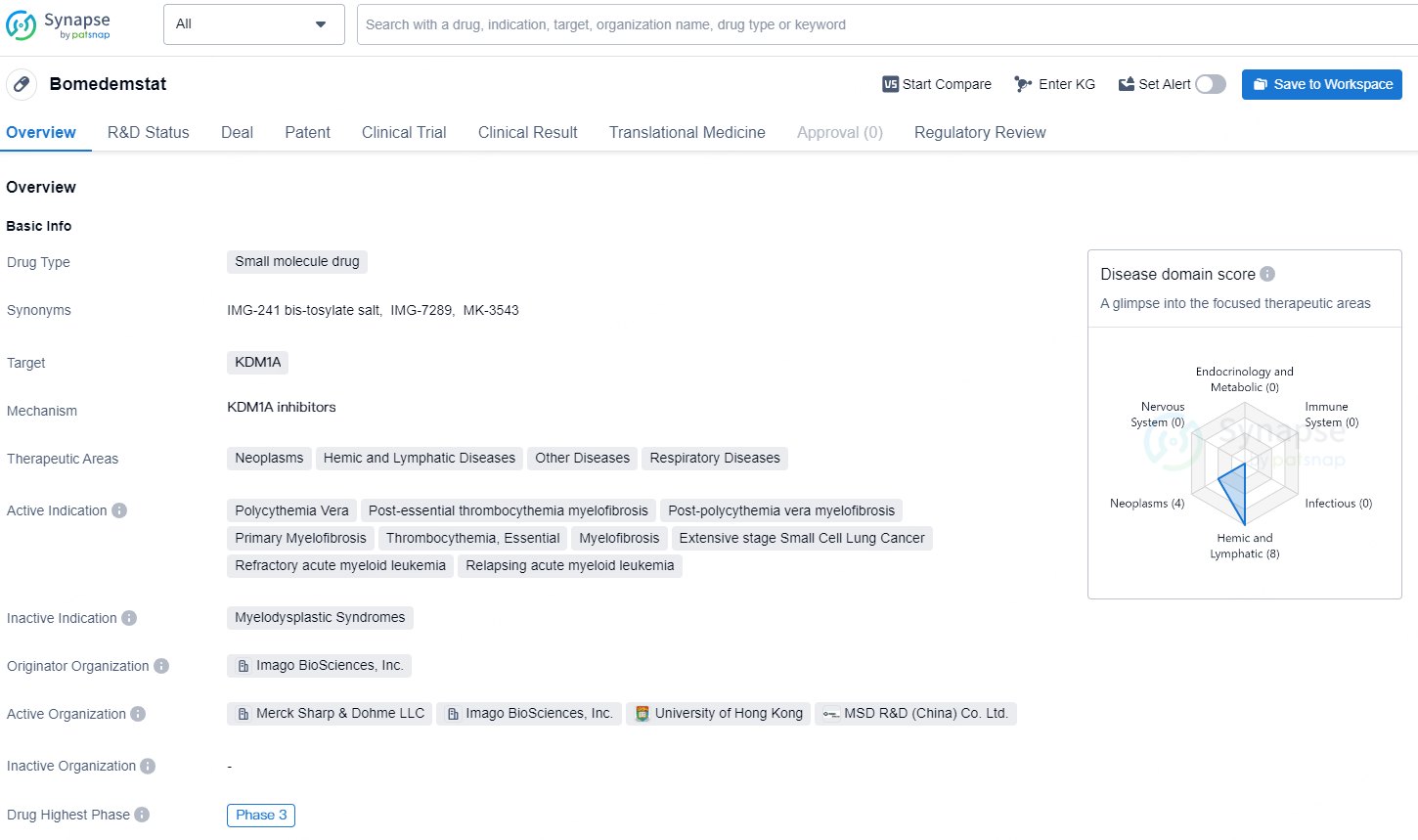
Bomedemstat is a small molecule drug designed to target KDM1A and is being developed by Imago BioSciences, Inc. The drug is intended to address therapeutic areas such as neoplasms, hemic and lymphatic diseases, other diseases, and respiratory diseases. Its active indications include polycythemia vera, post-essential thrombocythemia myelofibrosis, post-polycythemia vera myelofibrosis, primary myelofibrosis, thrombocythemia, essential, myelofibrosis, extensive stage small cell lung cancer, refractory acute myeloid leukemia, and relapsing acute myeloid leukemia.