Modalis Therapeutics Reports Promising Outcomes for Novel LAMA2-CMD Treatment, MDL-101
Modalis Therapeutics Corporation, a leading firm in creating novel medications for rare genetic disorders, employs its unique CRISPR-based epigenome editing technology named CRISPR-GNDM. Recently, the company shared a preprint on bioRxiv entitled “Efficient and durable gene activation by Cas9-mediated epigenome editing in vivo”. Modalis Therapeutics revealed findings that show significant durability, effectiveness, and safety in the dyW mouse model for LAMA2-CMD as well as in adult and juvenile non-human primates (NHPs).
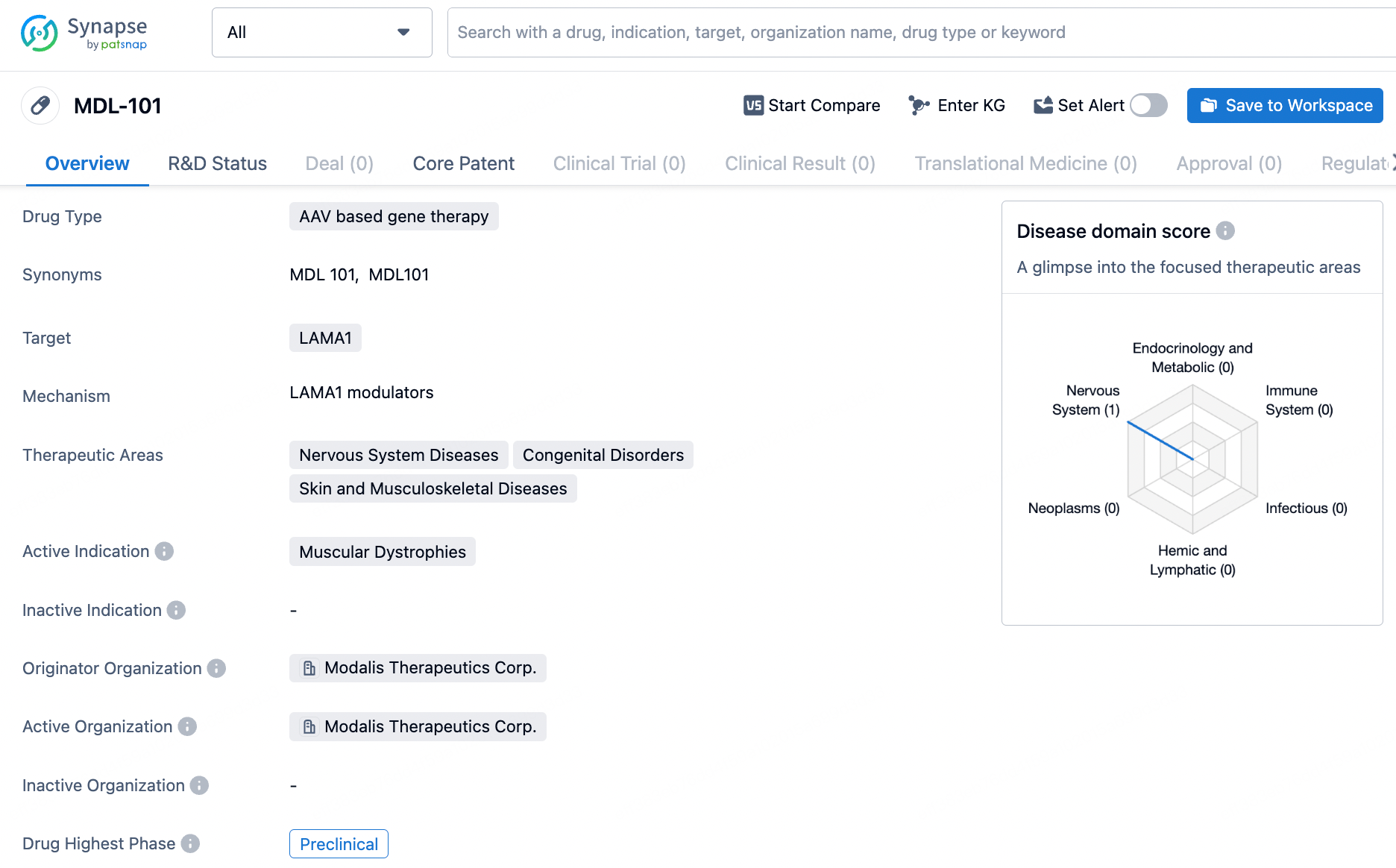
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
LAMA2-CMD, classified as a severe and early onset form of congenital muscular dystrophy, results from the deficiency of the LAMA2 protein. Despite substantial progress in the field of gene therapy, including the authorization of various treatments, the large size of the LAMA2-CMD gene, which consists of more than 3,000 amino acids, complicates traditional gene therapy strategies. These strategies usually employ AAV vectors to introduce a normal version of the affected gene, but the gene's size presents significant challenges.
As of now, there are no approved treatments that target the fundamental cause of LAMA2-CMD, nor are there any in advanced stages of clinical trials. However, Modalis Therapeutics has developed a unique CRISPR-GNDM technology that enables precise modulation of gene expression associated with the disease without causing double-stranded DNA breaks. Their pioneering candidate, MDL-101, aims to be a breakthrough therapy providing significant improvements for patients with LAMA2-CMD.
Haru Morita, the CEO of Modalis, expressed enthusiasm about the successful preclinical results: “We are excited to share our extensive preclinical data on MDL-101 via bioRxiv. This study is among the pioneering efforts showing effective systemic epigenome editing in non-human primates, demonstrating notable engagement with the target and boosting expression of the LAMA1 gene in muscle tissues."
MDL-101, currently under clinical investigation, is an innovative epigenetic editing treatment designed for LAMA2-Congenital Muscular Dystrophy. This therapy involves a modified, non-cutting Cas9 enzyme linked to a trans-activation domain, directed by a muscle-specific promoter within a muscle-targeted AAV vector. The treatment specifically targets the LAMA1 gene, a close relative of the LAMA2 gene, responsible for the disease.
The primary aim of MDL-101 is to enhance the expression of LAMA1 gene products in patient muscle tissue, compensating for the LAMA2 gene mutation's adverse effects. This approach has the potential to deliver a lasting, once-off treatment that could significantly improve the quality of life for those affected by LAMA2-CMD.
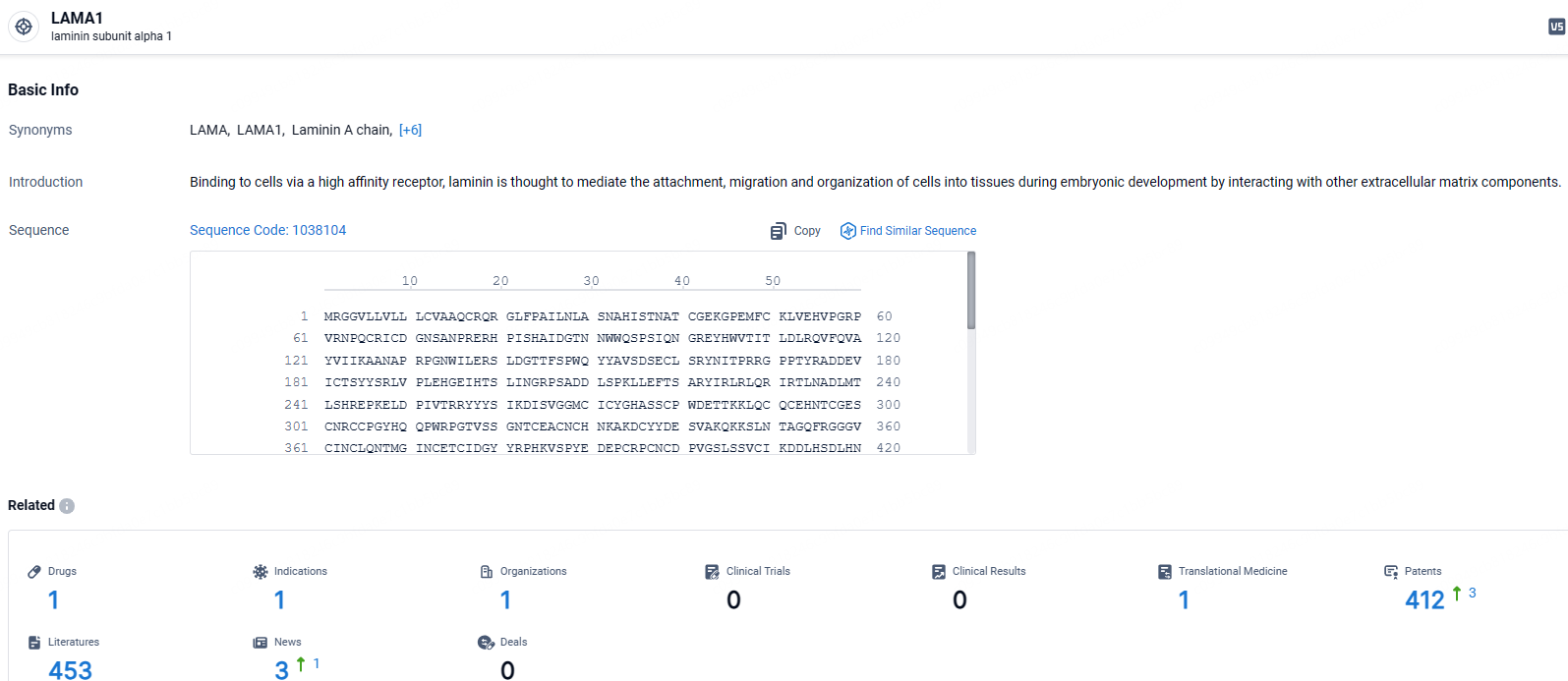
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 9, 2024, there are 1 investigational drugs for the LAMA1 targets, including 1 indications, 1 R&D institutions involved, and as many as 412 patents.
MDL-101 targets LAMA1 and is primarily focused on treating muscular dystrophies. Currently in the preclinical phase, MDL-101 holds promise for potential therapeutic interventions in various nervous system diseases, congenital disorders, and skin and musculoskeletal diseases. However, further research and clinical trials are required to determine its safety and efficacy in humans.