Nektar Therapeutics Presents Early Preclinical Results of TNFR2 Agonist NKTR-0165 at EULAR 2024
Nektar Therapeutics revealed the unveiling of a poster presenting novel preclinical insights on NKTR-0165 at the 2024 Congress held by the European Alliance of Associations for Rheumatology.
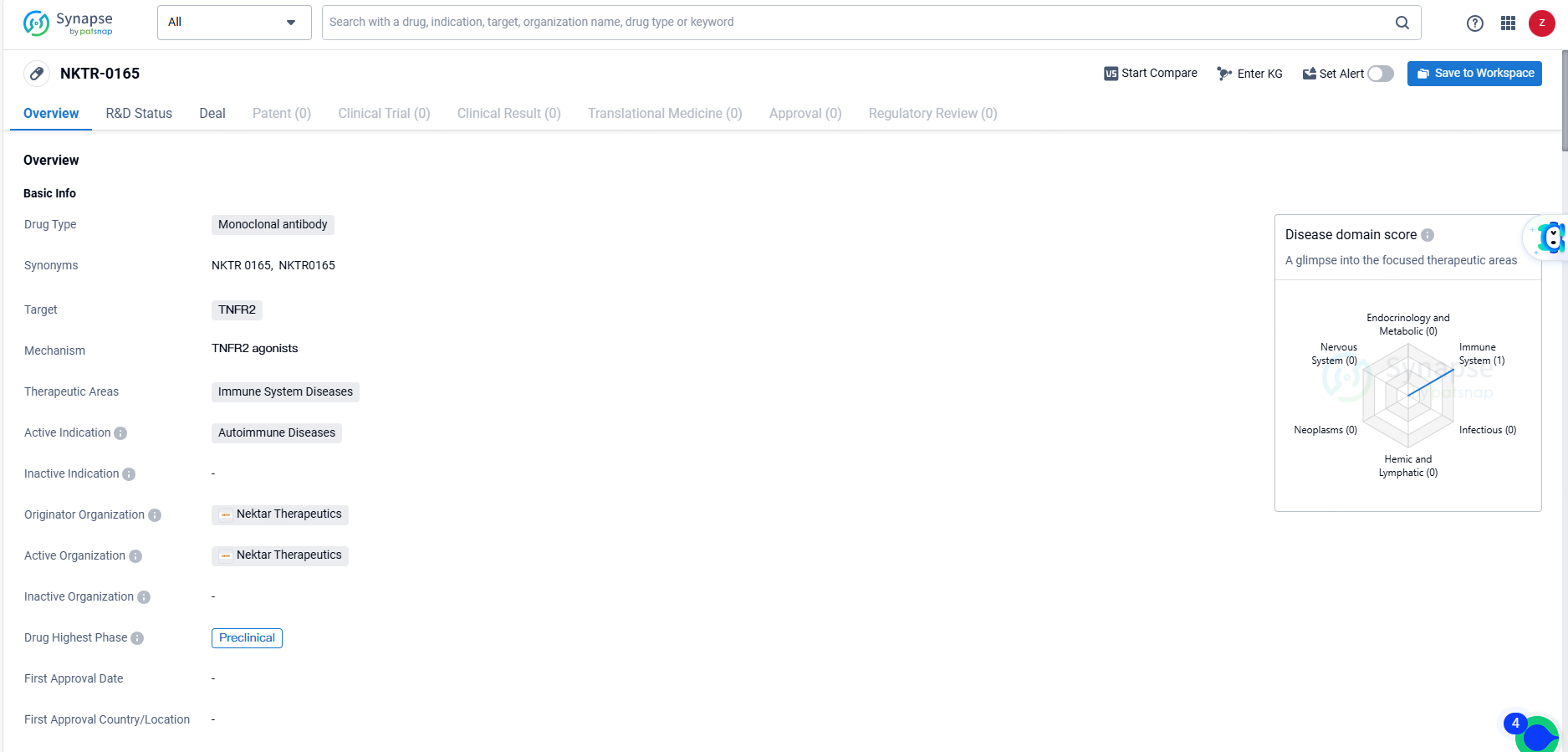
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
NKTR-0165 is an innovative and pioneering bivalent antibody that acts as an agonist for the tumor necrosis factor receptor 2 (TNFR2). It is specifically crafted to activate TNFR2 receptor activity without affecting TNFR1 signaling pathways. Research indicates that TNFR2 signaling plays a crucial role in regulating inflammation, and its deficiency or absence is linked to various autoimmune disorders. TNFR2 is prominently expressed on regulatory T cells (Tregs), neuronal cells, and endothelial cells, and it enhances the suppressive abilities and overall function of Tregs.
Jonathan Zalevsky, Ph.D., Senior Vice President and Chief Research & Development Officer at Nektar, stated, "The preclinical findings showcased at EULAR highlight that NKTR-0165 is a distinctive antibody that targets TNFR2 on Tregs to boost their immunosuppressive functions. This antibody holds promise as a potential first-in-class therapy for multiple autoimmune conditions, including ulcerative colitis and vitiligo."
Dr. Zalevsky further explained, "NKTR-0165 works by selectively binding to TNFR2, which upregulates the expression of critical proteins necessary for Treg proliferation and functionality. Animal studies demonstrate that NKTR-0165 mitigates inflammation by specifically enhancing the function of Treg cells through TNFR2 agonism. This data underscores the unique and differentiated profile of this bivalent antibody within the sphere of TNFR2 agonists."
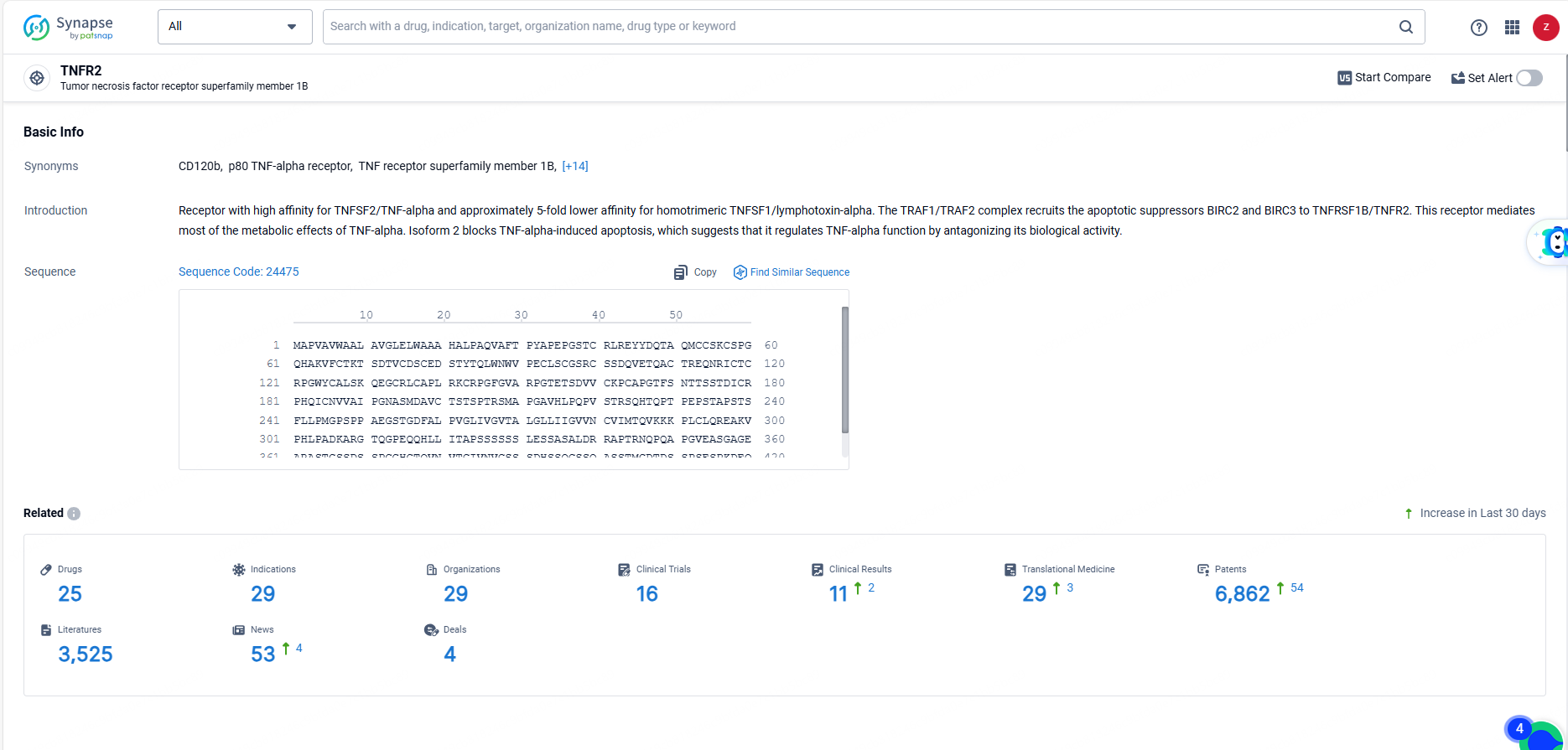
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 14, 2024, there are 25 investigational drugs for the TNFR2 target, including 29 indications, 29 R&D institutions involved, with related clinical trials reaching 11, and as many as 6862 patents.
NKTR-0165 represents a promising development in the field of biomedicine, with the potential to offer new treatment options for individuals with autoimmune diseases. However, it is important to note that the drug is still in the early stages of development, and further research and clinical trials will be necessary to determine its ultimate safety and effectiveness in human.