New Generation Vaccine Adjuvant: TLR Agonists
The term adjuvant derives from the Latin words ad and juvare, meaning "to help". The role of adjuvant is to help guide the vaccine antigen co-administered to generate the immune response required for protection. The development of new adjuvants has been at a standstill due to insufficient understanding of their mechanism of action. Recently, the discovery of Toll-Like Receptors (TLRs) has fundamentally transformed this field. TLRs are innate immune receptors that are responsible, directly or indirectly, for detecting pathogen-associated molecular patterns (PAMPs) and responding to them through the activation of innate and adaptive immune pathways.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Both naturally occurring and synthetic TLR agonists can leverage these endogenous immune signaling pathways to enhance and modulate vaccine responses, making them excellent vaccine adjuvants. This is significant not only for the development of vaccines against infectious diseases, but also for immunotherapies aimed at cancer, allergies, Alzheimer's disease and other disorders. Each TLR has its specific tissue localization and downstream gene signaling pathway, TLR agonists can be combined with other TLRs or alternative adjuvants to create combination adjuvants with synergistic or modulating effects, offering researchers the opportunity to precisely tailor adjuvants with specific immune effects.
TLR receptor family
The TLR receptor family includes six transmembrane TLRs (TLR-1, 2, 4, 5, 6, and 10) and four TLRs that are located within the endosome membrane (TLC-3, 7, 8, and 9). Each PAMP is recognized by different TLRs, i.e., lipopolysaccharides (TLR4), lipopeptides (TLR2 with TLR6 or TLR1), flagellin proteins (TLR5), single-stranded RNA (TLR7/8), double-stranded RNA (TLR3), and DNA containing CpG motifs (TLR9).
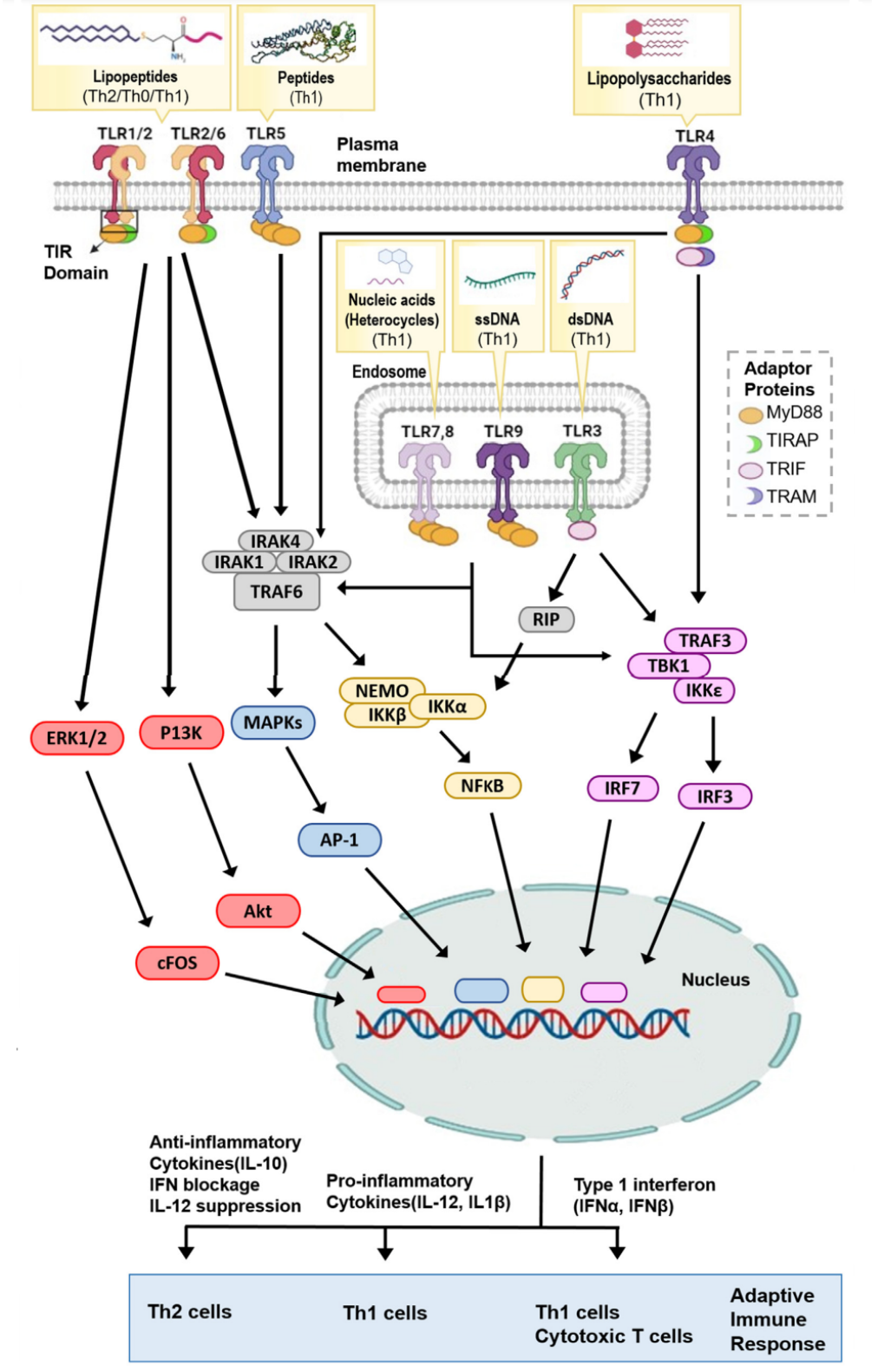
Upon encountering specific PAMPs, TLRs form homodimers (TLR3, TLR4, TLR5, TLR7, TLR8 and TLR9) or heterodimers (TLC1/2 or TLR2/6). Ligand-induced TLRs dimerization allows the adaptor protein MYD88 to bind to the TIR domain. TLR3 is unique in that it, as a universal adapter protein for TLRs, uses the TIR domain-containing interferon-β inducing protein (TRIF) as an adapter protein. The diversity of TLR receptors and adaptor proteins means that TLRs can participate in initiating many different downstream immune pathways.
TLR Signaling and Its Role in Immune Polarization
Activation of TLRs can regulate the type of immune responses generated by vaccines. The type of response is determined by the signaling pathways activated by specific TLRs and their adaptor proteins. Generally, most TLR pathways lead to a Th1 immune response, except for TLR2, which, possibly due to the diversity of TLR2 ligands, can induce pro-inflammatory and anti-inflammatory pathways, leading to Th1, Th0, or Th2 immune responses.
Except for TLR3, most TLRs function through MyD88. After TLRs are activated, MyD88 recruits a oligomeric complex composed of IL1 receptor-associated kinases (IRAK) 1, IRAK2, and IRAK4, and activates MyD88 tumor necrosis factor receptor associated 6 (TRAF6). The activated TRAF6 subsequently triggers the NF-κB and mitogen-activated protein kinase (MAPK) pathways, thereby inducing pro-inflammatory cytokines, such as IL-12 and TNF-α. TLR7 and TLR9 can activate the phosphorylation of interferon regulatory factor 7 (IRF7) by TRAF3 to produce IFN-α. Moreover, TLR3 and TLR4 have been shown to induce the production of IFN-β by recruiting TRAF3 through their TRIF adaptor proteins, thereby activating IRF3. Type I IFN responses can induce strong Th1 cell/cytotoxic T cell (CTL) responses, which are important for identifying and eliminating infected or cancer cells. TLR2 receptors mainly induce strong Th2 immune responses characterized by high IL-10 production and low IL-12.
TLRs do not specifically trigger Th1 or Th2 pathways, but can influence multiple pathways. It is the balance of signal events that determines immune bias. For example, one study showed that activation of TLR4 and TLR2 agonists can stimulate signal transmission through the p38 MAPK and ERK1/2-FOS pathways, leading to the production of IL-12 or IL-10, respectively. However, TLR4 induces p38 MAPK signal transmission at a higher threshold than TLR2, and TLR2 induces ERK1/2 signal transmission at a higher threshold than TLR4, resulting in immune polarization.
The Role of TLRs in Adaptive Immunity
TLRs coordinate many functions ranging from immune cell migration to enhancement of antibody affinity maturation. TLRs, acting as danger signal sensors, enhance the transportation of immune cells to the vaccine administration sites. TLRs participate in enhancing the antigen capture, processing, and presentation of both class I and II MHC molecules. Certain specific types of TLR can affect the cross-presentation of antigens to varying degrees, thus TLR3 or TLR4, not TLR2 or TLR7/8 ligands, reduce the uptake and cross-presentation of antigens by CD8 +T cells. TLR activation can also lead to upregulation of co-stimulatory molecules (such as CD80 and CD86), which is crucial for APCs to initiate naive T cells. TLRs of DC cells play a key role in self/non-self antigen recognition and in determining whether to produce an immune response or immune tolerance. Different types of APCs express different TLRs, such as TLR2 and TLR4 in myeloid DCs, whereas plasmacytoid DCs (pDCs) express TLR7 and TLR9. Therefore, TLRs' preferential activation of different DC lineages can affect the subsequent immune deviation.
Compared to mice, TLR expression on B cells is more limited in humans, mainly expressing TLR 2,7,9,10. TLR2 ligands require additional sensitization through the cross-linking of B cell receptors (BCR) and TLR7, and TLR7 requires type I interferon (IFN) actuation. These TLR agonists induce B cell proliferation, activation, and differentiation in vitro. Initially, it was believed that TLRs only promoted extrafollicular responses of B cells, characterized by rapid production of low-affinity antibodies. However, recent studies have shown that TLRs can also enhance germinal center reactions, thereby producing high-affinity antibodies. TLRs cooperate with BCRs to induce class-switch recombination and the maturation of antibody responses. TLR4 also enhances the migration of B cells to draining lymph nodes, and in this process, accelerates the transformation of antibody categories. The unsuitable B cell TLR signaling is related to the production of autoreactive B cells and autoimmune diseases; therefore, avoiding excessive TLR stimulation in vaccine formulations is very important.
It has also been shown that TLRs can enhance various different T cell subsets. Several studies have shown that TLRs can serve as co-stimulatory molecules for CD8+T cells, promoting cell proliferation/survival, effector function, cytokine production, and memory formation. The role of TLRs in Treg cells remains controversial. Some studies have shown that TLR2 agonists can induce a temporary loss of Treg inhibitory activity, whereas other studies have shown that despite TLR2 agonists increasing antigen-specific proliferation, Treg cells still retain their inhibitory function.