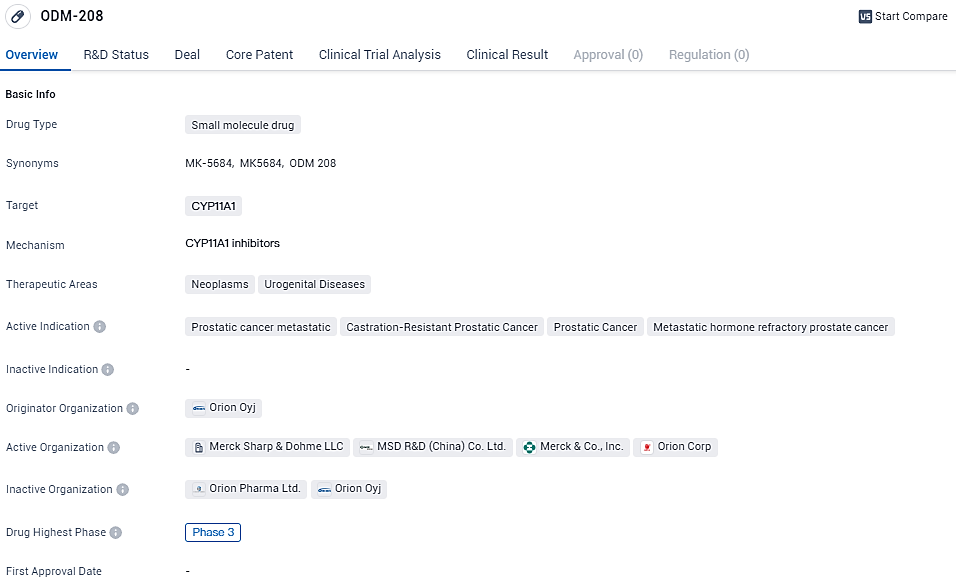
New Phase II Findings for ODM-208/MK-5684 Revealed at 2024 ASCO-GU Conference
During the 2024 ASCO Genitourinary Cancers Symposium, Orion showcased a new poster, offering further insights derived from the Phase II CYPIDES study. This research is meticulously assessing the tolerability and clinical outcomes of ODM-208 (also designated as MK-5684), a trial-phase oral CYP11A1 inhibitor. The focus is on patients who have undergone extensive prior treatments and are now confronting advanced metastatic castration-resistant prostate cancer, encompassing cases both with and without mutations in the androgen receptor ligand-binding domain (AR-LBD).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Past literature predominantly examined androgen receptor (AR) gene mutations within the ligand-binding-domain (LBD). The latest findings provide preliminary insights from a supplementary group comprised mostly of AR-LBD wild-type cases, which enhances the earlier phase 2 findings.
Even when prostate cancer progresses to a stage that is unresponsive to castration, steroid hormones remain influential in its control. The early outcomes from the Phase II CYPIDES study indicate the robust efficacy of ODM-208/MK-5684 in halting the production of all steroid hormones, exhibiting notable therapeutic effects in extensively treated metastatic castration-resistant prostate cancer (mCRPC) subjects, notably those with AR-LBD mutations.
"It's quite compelling that those patients who are AR-LBD wild-type seem to gain advantages from using ODM-208/MK-5684 as well, although the decline in PSA levels was more commonly observed in individuals with the AR-LBD mutation. We are eager to uncover more comprehensive results from both AR-LBD positive and negative cohorts in the forthcoming Phase 3 trials," stated Professor Outi Vaarala, M.D., Ph.D., who functions as the Senior Vice President of Innovative Medicines and Research and Development at Orion.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
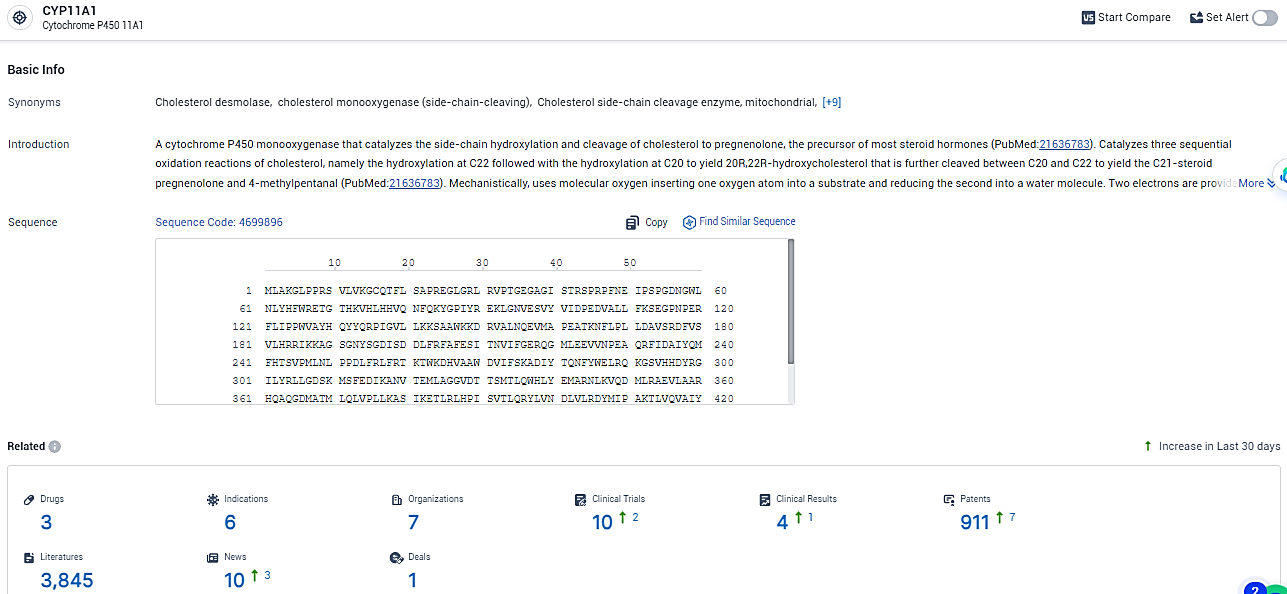
According to the data provided by the Synapse Database, As of January 31, 2024, there are 3 investigational drugs for the CYP11A1 target, including 6 indications, 7 R&D institutions involved, with related clinical trials reaching 10, and as many as 911 patents.
ODM-208/MK-5684 is an investigational oral, non-steroidal and selective inhibitor of the CYP11A1 enzyme discovered and developed by Orion for the treatment of hormone-dependent cancers, such as prostate cancer. The therapeutic areas of neoplasms and urogenital diseases highlight the potential applications of ODM-208 beyond prostate cancer.