Nkarta Begins Investigator-Led NKX019 Clinical Trial for Systemic Lupus Erythematosus
Nkarta, Inc., a biopharmaceutical firm focusing on the development of engineered natural killer cell therapies, declared that researchers from Columbia University Irving Medical Center have commenced an investigator-initiated trial involving NKX019. This therapy, NKX019, is an allogeneic, CD19-targeted chimeric antigen receptor NK-cell treatment, curated by Nkarta for individuals diagnosed with systemic lupus erythematosus.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“Individuals with lupus encounter significant hurdles, including prolonged exposure to toxic or partially effective medications,” stated Dr. Askanase. “Cell therapy offers the promise of achieving long-term remission without the need for ongoing medication.”
The CUIMC IST aims to enroll up to six patients with SLE, irrespective of renal involvement, to assess safety and clinical outcomes in a potentially different cohort than Ntrust-1. Planned translational and biomarker studies will include autoantibodies, cytokine profiles, and pharmacokinetics. Patients will receive NKX019 on Days 0, 7, and 14 following single-agent lymphodepletion with cyclophosphamide. Patient recruitment is currently in progress.
Systemic lupus erythematosus is an autoimmune condition in which the body's immune system attacks its own tissues. This dysregulation leads to the production of antibodies that can damage various organs such as the skin, joints, kidneys, heart, and brain. Symptoms can vary from fatigue and joint pain to severe, life-threatening organ damage. SLE can lead to lupus nephritis, a serious kidney complication.
“With its reduced-toxicity lymphodepletion regimen, achieved through cytokine engineering, we believe NKX019 holds the potential to benefit a broader range of patients, including those with less severe disease,” said David R. Shook, M.D., Nkarta’s Chief Medical Officer and Head of R&D. “We are excited to work with our academic partners at Columbia and are committed to impacting the lives of those living with autoimmune diseases such as lupus.”
NKX019 is an allogeneic, cryopreserved, off-the-shelf immunotherapy candidate that utilizes natural killer cells sourced from the peripheral blood of healthy adult donors. It is engineered with a humanized CD19-directed chimeric antigen receptor for improved cell targeting and a proprietary, membrane-bound form of interleukin-15 (IL-15) for better persistence and activity without the need for external cytokine support. CD19 serves as a biomarker for normal B cells as well as those involved in autoimmune diseases and B cell-derived cancers.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
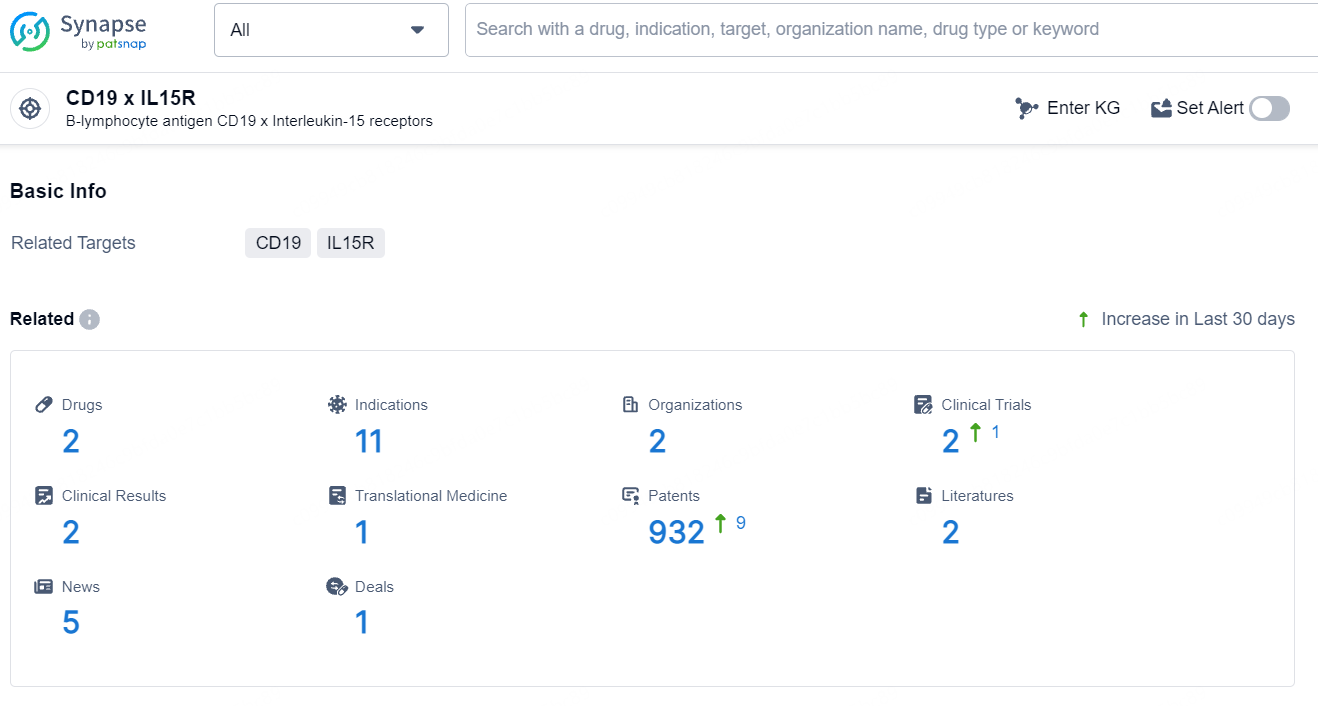
According to the data provided by the Synapse Database, As of July 31, 2024, there are 2 investigational drugs for the CD19 and IL15R target, including 11 indications, 2 R&D institutions involved, with related clinical trials reaching 2, and as many as 932 patents.
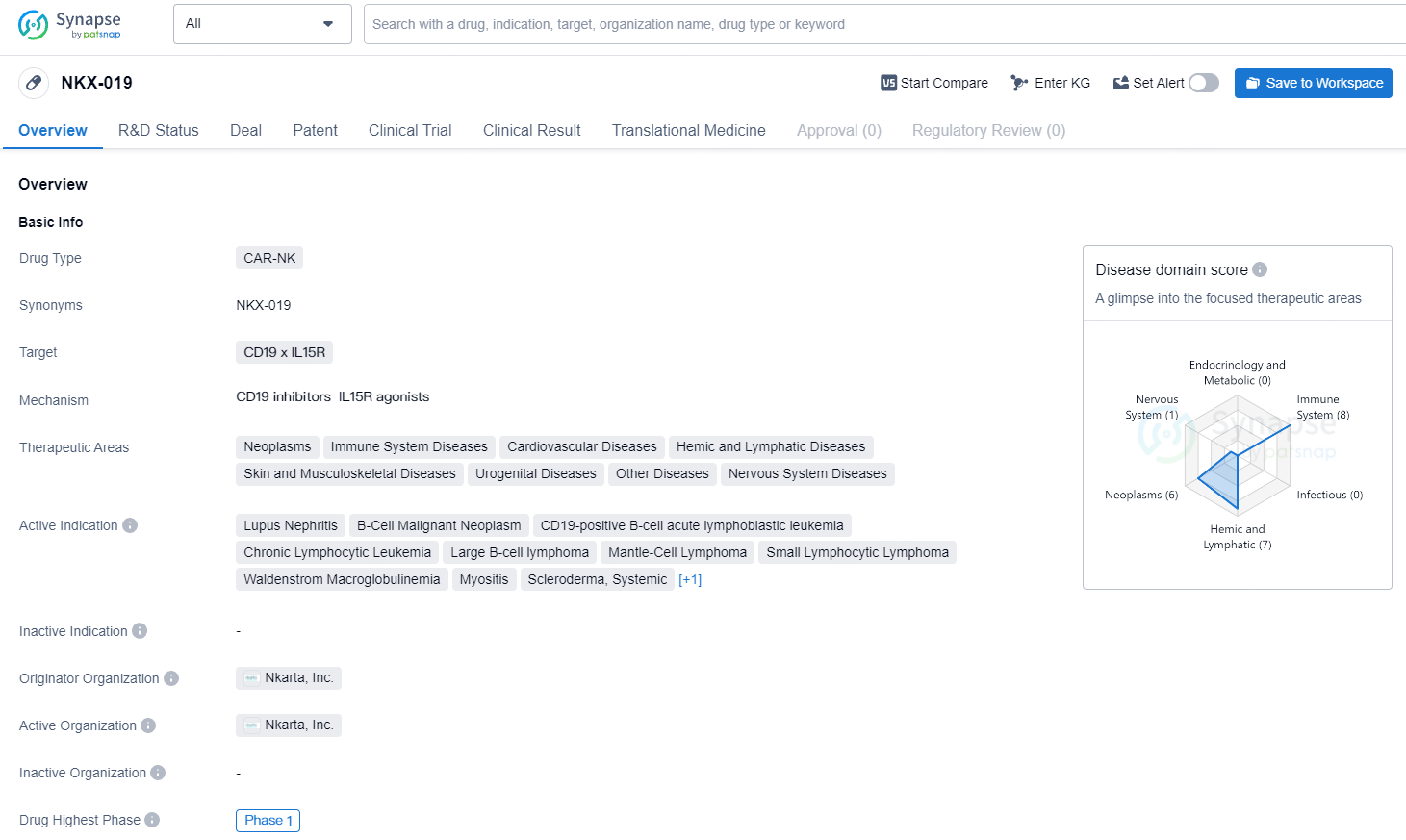
NKX-019 is a CAR-NK drug targeting CD19 x IL15R, with active indications for a wide range of diseases, particularly in the field of cancer and immune system disorders. Its development is currently in Phase 1, and it has the potential to address unmet medical needs in these therapeutic areas.