Novo Nordisk plans to purchase Cardior Pharmaceuticals, bolstering its portfolio in heart health management
Novo Nordisk has entered into an acquisition agreement with Cardior Pharmaceuticals, with the deal amounting to a maximum of 1.025 billion Euros. This sum encompasses an initial cash outlay and further conditional increments based on the attainment of specific developmental and sales benchmarks.
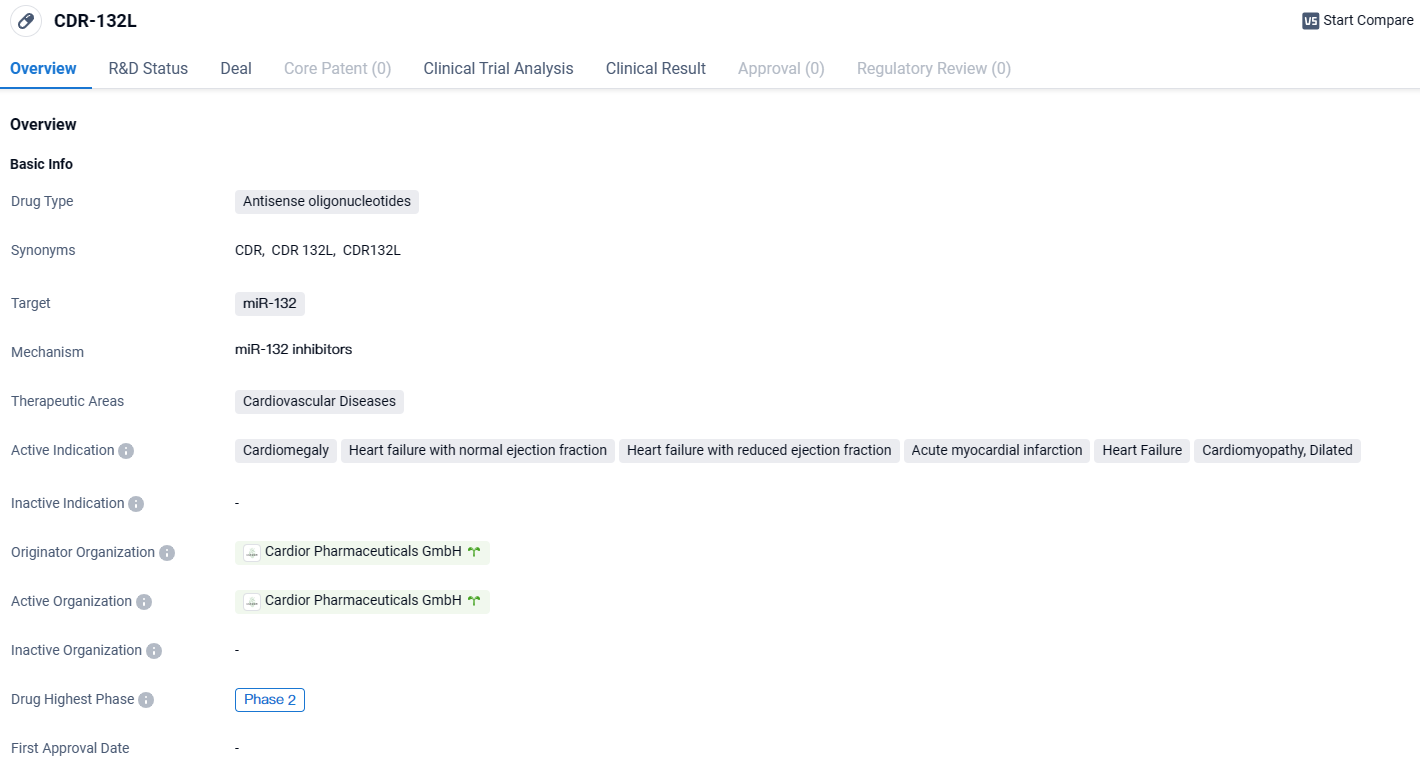
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Headquartered at the forefront of pioneering cardiovascular treatments, Cardior is renowned for its innovative strategies in the creation and refinement of treatments using RNA manipulation to combat, mend, and potentially reverse cardiovascular illnesses. The company adopts a novel technique, targeting specific non-coding RNAs, as its foundation for confronting the underlying mechanisms of heart anomalies, with the ultimate goal of delivering enduring benefits to patients.
The deal encompasses the inclusion of Cardior's flagship agent, CDR132L, which is presently navigating through phase 2 trials, focusing on heart failure therapies.
For Novo Nordisk, the integration of Cardior represents a significant progression in its ambition to carve out a significant role within the realm of cardiovascular illnesses. Novo Nordisk is on a mission to amass a potent and specific therapy collection both through internal innovation and strategic collaborations to meet the cardiovascular sector's pressing and underserved demands—the leading culprit behind global mortality rates.
"Cardior's incorporation into Novo Nordisk's framework promises to bolster our series of cardiovascular ventures, complementing our existing array of projects at varying stages of clinical progress," mentioned Martin Holst Lange, Novo Nordisk's executive vice president for Development.
"The expert scientific endeavors of the Cardior team have left us exceedingly impressed, particularly with CDR132L's unique operating mechanism and its potential to pioneer as a category-defining treatment capable of arresting or even reversing disease progression in individuals grappling with heart failure," he further stated.
CDR132L has been meticulously engineered to check and counteract cellular anomalies by specifically inhibiting the aberrant microRNA molecule miR-132, thereby fostering potential enhancements in cardiac performance with lasting effects.
Claudia Ulbrich, MD, the CEO and co-founder of Cardior, remarked, "The acquisition mirrors the transformative capabilities inherent in CDR132L as a groundbreaking therapeutic option for heart failure management." She expresses confidence in Novo Nordisk's suitability as a partner, given its extensive experience in clinical offerings and commercial knowledge, coupled with the resources to expedite our advanced development stages, including expansive registration-targeted studies. Ulbrich anticipates eagerly the progression of CDR132L toward regulatory approval.
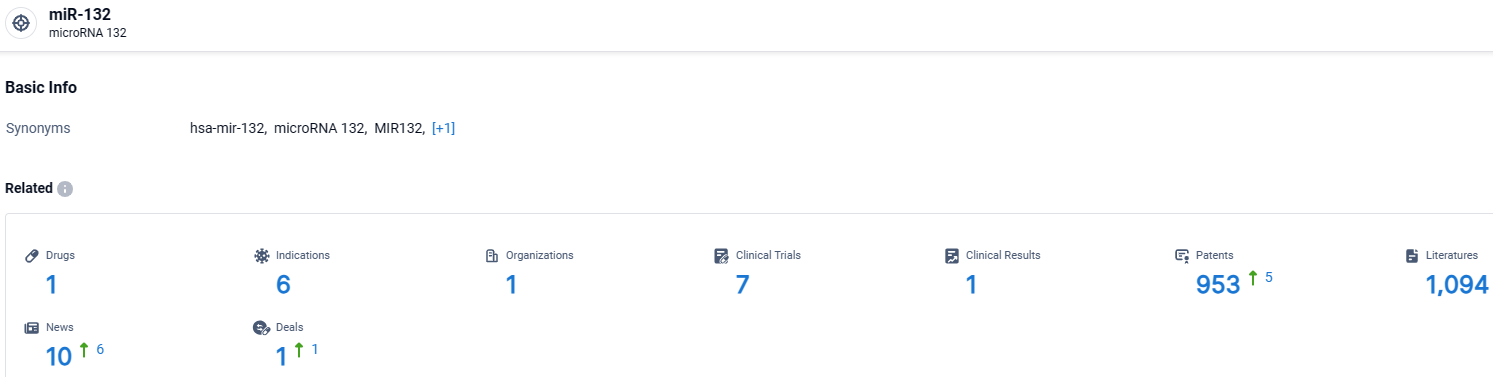
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 27 2024, there are 1 investigational drugs for the miR-132 target, including 6 indications, 1 R&D institutions involved, with related clinical trials reaching 7, and as many as 953 patents.
CDR-132L targets miR-132 in cardiovascular diseases. With its highest phase of development being Phase 2, this drug holds promise in addressing conditions such as cardiomegaly, heart failure, and cardiomyopathy. Further research and clinical trials will be needed to determine its overall efficacy and safety.