OBI Pharma Declares FDA Approval of Investigational New Drug Application for Early-Stage OBI-992 Trial in the U.S
OBI Pharma, a firm specializing in the development of cancer treatments currently in clinical evaluation, has made public that the FDA in the United States has granted authorization for a new drug exploration application regarding OBI-992, permitting the commencement of an early to mid-stage clinical trial to assess the efficacy of their innovative cancer treatment. This treatment is a cutting-edge antibody-linked drug specifically aimed at the TROP2 antigen.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
OBI has outlined plans to initiate patient recruitment for its upcoming clinical study, focusing on a suite of advanced solid malignancies, including non-small cell lung cancer, small cell lung cancer, and gastric cancer. Alongside these, a series of additional cancers are being considered for the trial. The company's Chief Medical Officer, Wayne Saville, M.D., highlighted that the key aims of the trial are to scrutinize the safety profile, analyze the pharmacokinetic aspects, and gain initial insights into the effectiveness of OBI-992, an innovative TROP2 ADC that posits itself as a leader in its class. Anticipation surrounds the administration of OBI-992 to the first participant in the upcoming Phase 1/2 trial planned for early 2024.
In a further statement, Heidi Wang, Ph.D., the Chief Executive Officer at OBI Pharma, mentioned the distinctiveness of OBI-992, which is a product of OBI's own research and development. This TROP2 ADC has shown superior results in preclinical trials, especially in efficacy, safety, and stability against its competitors. The excitement is palpable within OBI Pharma as they prepare to launch the inaugural human trials with OBI-992 and are committed to pushing forward their advanced therapies into clinical settings for the benefit of those suffering from cancer.
OBI-992 stands out as a TROP2-targeted ADC, carrying a powerful payload of a topoisomerase I inhibitor, specifically designed to eradicate cancer cells. TROP2 expression is prevalent across a wide array of solid tumor forms such as those of the lung, breast, ovarian, and gastric cancers, which positions TROP2 as an optimal target for oncological treatments.
Featuring a specialized hydrophilic linker that is cleaved by enzymes, OBI-992 is crafted to remain stable in the bloodstream yet is capable of delivering its potent anti-cancer payload directly inside cancer cells. The evidence from animal studies indicates that OBI-992 not only provides substantial tumor suppression but also exhibits enhanced pharmacokinetic attributes and a promising safety profile.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
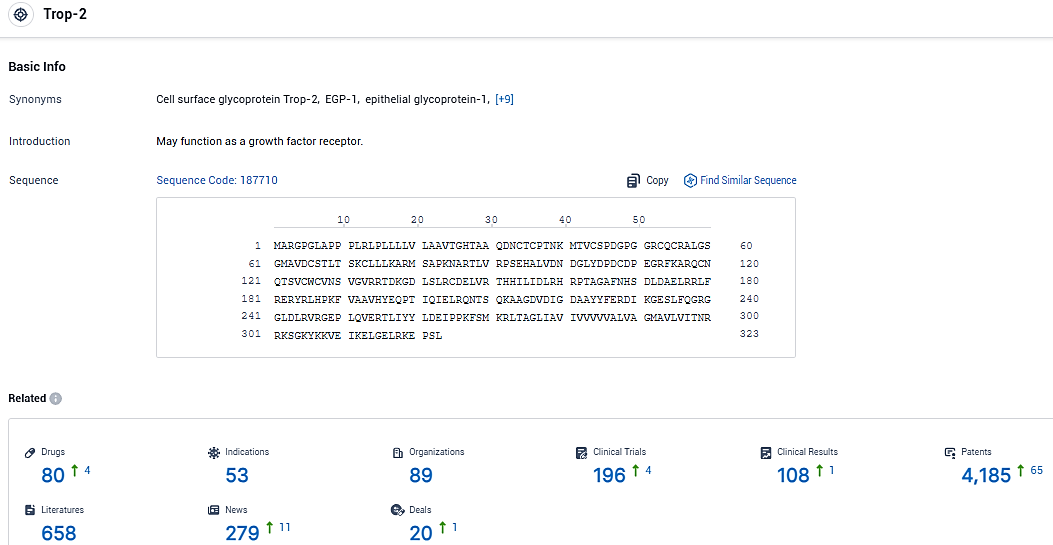
According to the data provided by the Synapse Database, As of January 6, 2024, there are 80 investigational drugs for the Trop-2 target, including 53 indications, 89 R&D institutions involved, with related clinical trials reaching 196, and as many as 4185 patents.
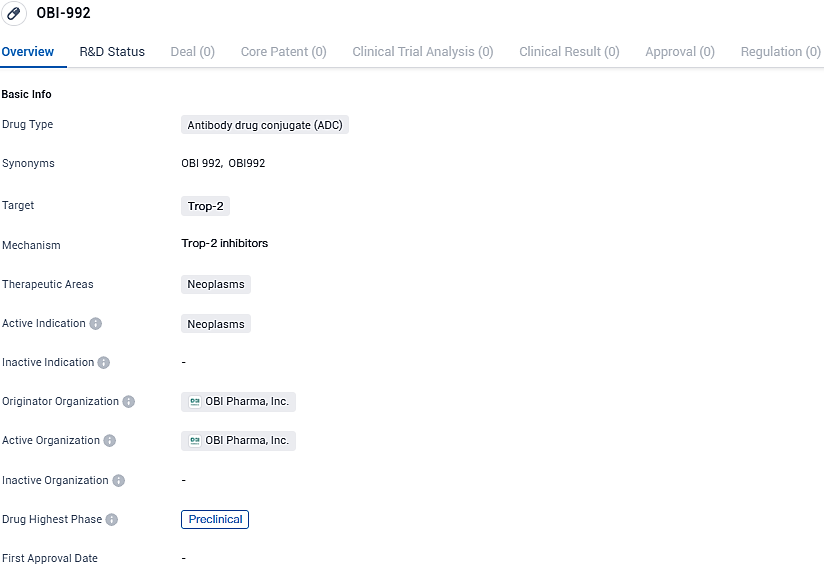
OBI-992 is currently in the preclinical phase of development globally and in China. The potential of ADCs in improving cancer treatment has generated significant interest, and further research is needed to determine the safety and efficacy of OBI-992 in clinical trials.