Oculis Starts Phase 2b RELIEF Trial Enrolling First Patient for Licaminlimab (OCS-02), a Topical Anti-TNFα Drug for Dry Eye Treatment
Oculis Holding AG, a worldwide biopharma firm dedicated to preserving vision and advancing ophthalmologic treatment, has declared the initiation of its Phase 2b RELIEF study with the enrollment and initial consultation of its first participant. The study is focused on assessing the efficacy of licaminlimab (also known as OCS-02). This cutting-edge anti-TNFα biologic is administered as an eye drop and is being tested for its effectiveness in combating the symptoms of Dry Eye Disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The stage 2b RELIEF study involves a collaborative effort across different sites, employing a random allocation, blinded and placebo-controlled methodology to assess both the safety profile and therapeutic potential of licaminlimab in alleviating the clinical manifestations associated with moderate to severe Dry Eye Disease (DED). This study will also investigate whether individuals who carry a particular genetic marker, identified in a preceding study, show an enhanced response to licaminlimab.
Design of the study was informed by encouraging results from preceding studies of licaminlimab used in both DED and Uveitis. It is planned that 120 participants will be assigned in a random fashion to receive either licaminlimab or a placebo. The treatment duration is set for six weeks, with an additional two weeks of observation. We expect to summarize the initial findings by the middle of 2024.
Eric Donnenfeld, M.D., a respected faculty member specializing in Ophthalmology at New York University as well as a co-leader of the Oculis Scientific Advisory Board, remarked, "Licaminlimab stands out as an innovative therapy applied directly to the eye and has demonstrated greater effectiveness than placebo in mitigating the eye discomfort experienced by patients with severe DED, and is also acknowledged for its favorable tolerance levels. A particularly notable discovery from an earlier Phase 2a study was the identification of a genetic marker that may enable us to pinpoint patients who will have an optimal response to licaminlimab, an aspect we are excited to further examine in the ongoing RELIEF study.”
Added insights were shared by Christophe Baudouin, M.D., a distinguished authority in Ophthalmology and the department head at Quinze-vingts National Ophthalmology Hospital, who stated, “Licaminlimab makes use of the already established anti-TNFα action pathway which has the potential to be extremely advantageous, considering the pivotal role that inflammation of the ocular surface plays in DED. I am of the opinion that the anti-inflammatory and cell-protective properties that result from the inhibition of TNFα have the potential to substantially enhance the treatment approach for a number of eye conditions marked by inflammation.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
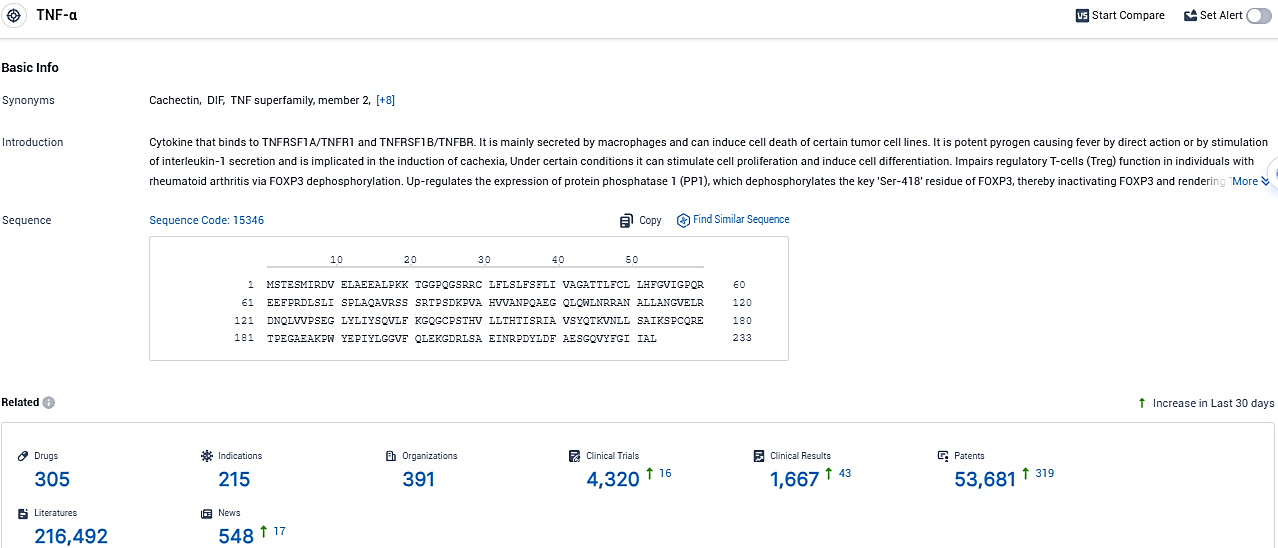
According to the data provided by the Synapse Database, As of December 15, 2023, there are 305 investigational drugs for the TNF-α target, including 215 indications, 391 R&D institutions involved, with related clinical trials reaching 4320, and as many as 53681 patents.
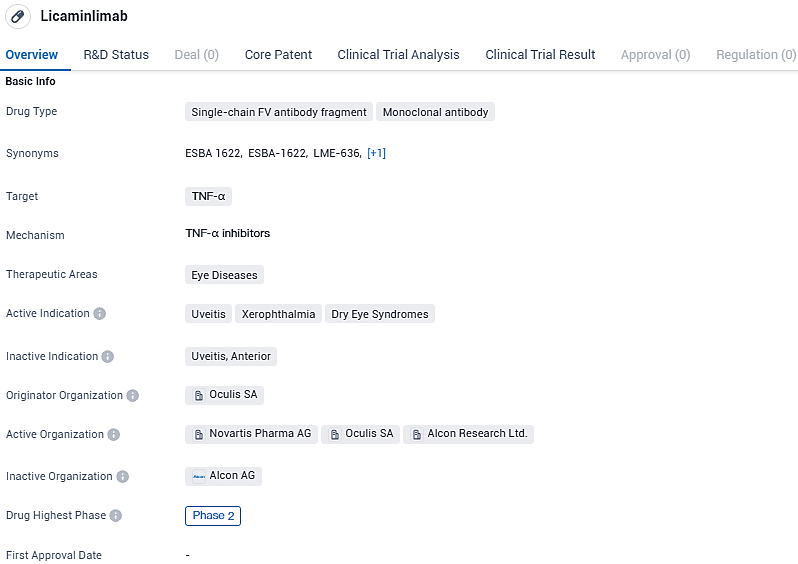
Licaminlimab is a single-chain FV antibody fragment and monoclonal antibody. It targets TNF-α and is intended for the treatment of uveitis, xerophthalmia, and dry eye syndromes. As of the latest information, it has reached Phase 2 of development.