Orchard Therapeutics Declares Approval of Biologics License Application for OTL-200 pertaining to MLD
Orchard Therapeutics, a renowned leader in gene therapy worldwide, reported that the U.S. FDA has approved the submission of their BLA for OTL-200, which is for metachromatic leukodystrophy, prioritizing its investigation. A target date of March 18, 2024, has been determined by the agency under the Prescription Drug User Fee Act.
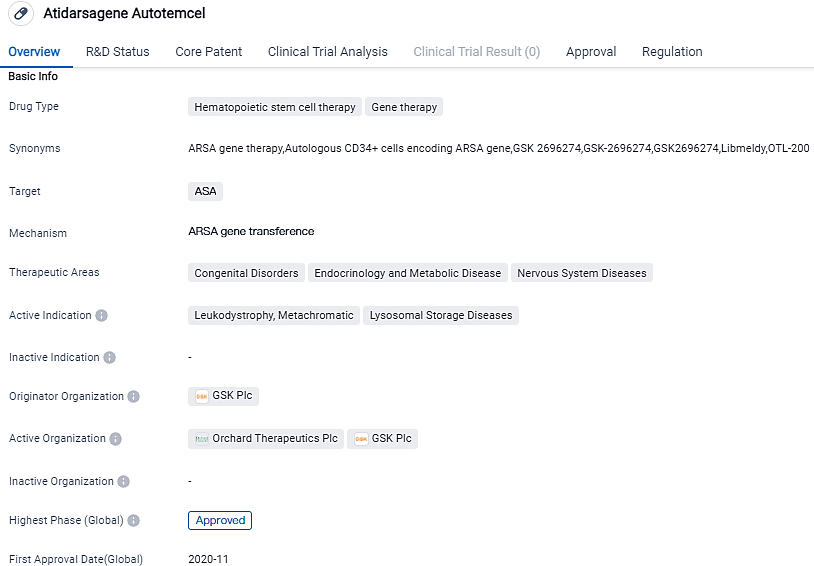
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"This is another significant advancement for the U.S patients and their families grappling with this heart-wrenching and ruthless illness which for a long stretch has put them under the unbearable strain of coping with the diagnostic journey, receiving information that there were no treatments exceeding palliative care, and then witnessing their child's condition deteriorate," remarked Bobby Gaspar, M.D., Ph.D., co-founder and CEO of Orchard Therapeutics.
Dr. Gaspar continued, “Contemplating on the remarkable progress we have collectively achieved in the brief eight months since our Type B clinical consultation with the FDA, I would like to use this opportunity to convey my deepest appreciation to the Orchard team. Although there remains more work to be undertaken, it's through your hard work we are turning our communal vision of ending the devastation brought about by severe genetic diseases into a reality for MLD.”
OTL-200 had previously been awarded both the Rare Pediatric Disease and Regenerative Medicine Advanced Therapy designations by the FDA and has been approved as Libmeldy®(atidarsagene autotemcel) by the EC and UK Medicines and Healthcare products Regulatory Agency.
MLD is a rare and life-threatening inherited disease of the body’s metabolic system, believed to happen in approximately one in every 100,000 live births according to existing publications. MLD is triggered by a mutation in the arylsulfatase-A (ARSA) gene resulting in sulfatides' accumulation in the brain and other parts of the body such as the liver, gallbladder, kidneys, and/or spleen.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 25, 2023, there are 11 investigational drugs for the ASA target, including 3 indications,14 R&D institutions involved, with related clinical trials reaching 17,and as many as 7149 patents.
Libmeldy (atidarsagene autotemcel), also known as OTL-200, has been approved by the European Commission for the treatment of MLD. OTL-200 is indicated for the treatment of leukodystrophy, metachromatic, and lysosomal storage diseases. Its regulatory designations emphasize its potential to address rare pediatric diseases, undergo priority review, and utilize advanced regenerative medicine techniques.