Orchard Therapeutics Gains FDA Approval for Lenmeldy™ (atidarsagene autotemcel) for Early-Stage Metachromatic Leukodystrophy in Children
Orchard Therapeutics recently disclosed that the U.S. Food and Drug Administration has sanctioned Lenmeldy™ (atidarsagene autotemcel), previously identified as OTL-200, as a therapeutic agent for young patients detected with early-stage Metachromatic Leukodystrophy (MLD) in its various forms, specifically pre-symptomatic late infantile, pre-symptomatic early juvenile, or those exhibiting early signs of the early juvenile phase. This group is commonly described as having early-onset MLD.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The sanctioning of Lenmeldy by the FDA ushers in significant new opportunities for American children suffering from early-stage MLD. These individuals had no other alternatives except palliative and terminal care," stated Orchard Therapeutics' co-founder and CEO, Bobby Gaspar, M.D., Ph.D.
Dr. Gaspar added, "The unwavering dedication of our team at Orchard has been pivotal in reaching this milestone. We are committed to facilitating the dissemination of this groundbreaking treatment to those patients who stand to benefit from it the most."
MLD, a devastatingly rare and lethal inherited condition, stems from a mutation in the gene that is crucial for producing the arylsulfatase A enzyme. This mutation causes significant nerve system damage and the regression of developmental abilities. This is due to the buildup of brain and body sulfatides, which can severely damage neural function over time if not properly degraded.
The most severe cases of MLD see children, who initially develop without indication of the disorder, suddenly and progressively losing core abilities such as moving, speaking, and engaging with their environment. These young patients may eventually enter a vegetative state requiring constant, round-the-clock, intensive care. The prognosis is dire, with most losing their lives within half a decade from the onset of the condition, which inflicts psychological and economic strain upon their families.
The therapeutic goal of Lenmeldy is to rectify the genetic root of MLD by the insertional transfer of healthy ARSA gene copies into a patient's hematopoietic stem cells extracorporeally, through a lentiviral vector. This innovative method has shown promise in re-establishing enzymatic activity, potentially halting or decelerating the advancement of the disease after just one application.
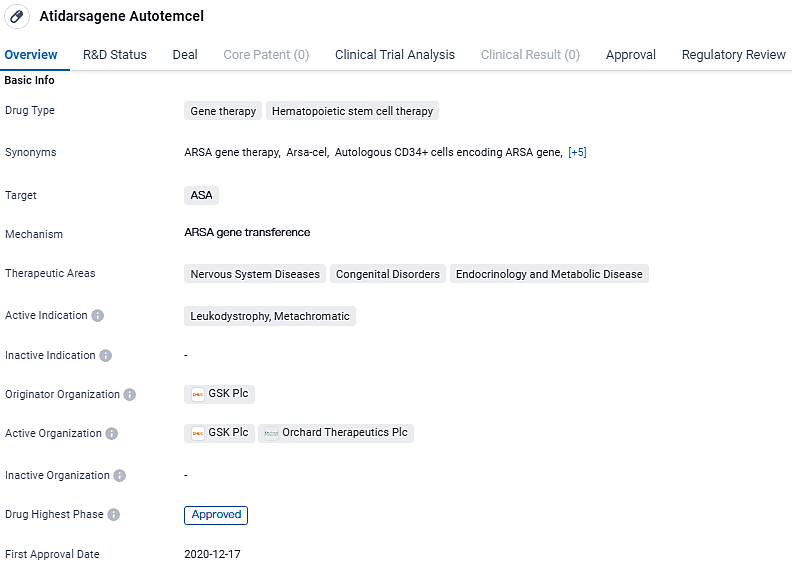
In September 2023, Lenmeldy received Priority Review status. It had already been awarded the Rare Pediatric Disease and Regenerative Medicine Advanced Therapy designations by the FDA. Following this approval, Orchard Therapeutics obtained a Priority Review Voucher, set to be conveyed to GSK in line with the predefined conditions of their initial license arrangement.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
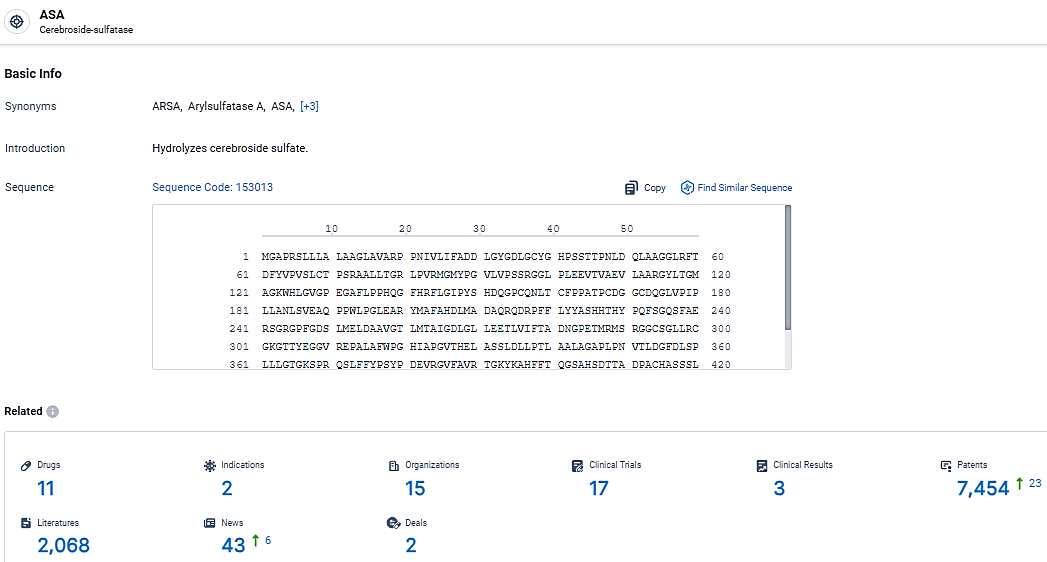
According to the data provided by the Synapse Database, As of March 20, 2024, there are 11 investigational drugs for the ASA target, including 2 indications, 15 R&D institutions involved, with related clinical trials reaching 17, and as many as 7454 patents.
Atidarsagene Autotemcel has been approved for the treatment of Leukodystrophy, Metachromatic, and has shown promise in addressing various nervous system diseases, congenital disorders, and endocrinology and metabolic diseases. The drug has undergone rigorous regulatory processes, including Priority Review, Regenerative Medicine Advanced Therapy designation, and Rare Pediatric Disease designation. Its approval in multiple countries signifies its potential to provide significant therapeutic benefits to patients in need.