Overview of the Development Progress of TIGIT Drug Target
TIGIT, a major immune checkpoint molecule, is expressed on the surface of T cells and natural killer (NK) cells.
In 2002, researchers from Genentech first discovered this gene, which exhibited specific expression in T cells and NK cells. Subsequently, TIGIT made its debut in 2009 in Nature Immunology, where it was identified to possess an immunoglobulin-like (IgV) domain and an immunoreceptor tyrosine-based inhibition motif (ITIM). Scientists thus named it TIGIT (T-cell immunoreceptor with Ig and ITIM domains).
TIGIT plays a significant role in suppressing T cells and NK cells.
Further studies revealed that TIGIT belongs to the poliovirus receptor (PVR)/Nectin family, serving as a co-inhibitory receptor expressed in lymphocytes. It is highly expressed on effector and regulatory CD4+ T cells, follicular helper CD4+ T cells, and NK cells, among others, thereby inhibiting the function of these immune cells.
Moreover, research has found that TIGIT is expressed on NK cells in most tumors, and its role in tumor immune suppression is similar to that of PD-1/PD-L1. The combined use of TIGIT inhibitors and PD-1/PD-L1 inhibitors can synergistically exert anti-tumor effects.
It is worth noting that, unlike the typical one-to-one or one-to-many relationships between most known immune checkpoints and their ligands, TIGIT maintains a "many-to-many" relationship with its ligands CD226, CD96, CD112, and CD155.
This complex relationship contributes to the incomplete understanding of TIGIT's mechanism, posing one of the major challenges in TIGIT research and development.
Competitive Landscape
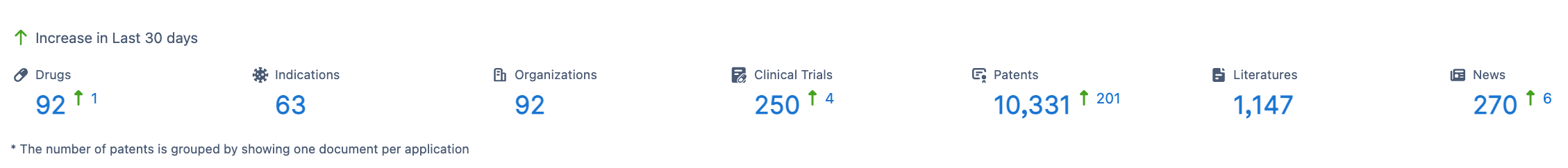
According to Synapse database, there are currently 92 drugs targeting TIGIT in various stages of development.
These drugs target a wide range of indications, covering 58 different disease types. A total of 92 research organizations are involved in the development of TIGIT-targeted therapies. Clinical trial data reveals that there have been 250 clinical trial results associated with TIGIT-targeted drugs.
Additionally, the development of TIGIT-targeted drugs has garnered significant attention and protection, with a total of 10,331 patent applications related to TIGIT-targeted therapies. To support further advancements in TIGIT-targeted drug research, there have been 1,147 published scientific articles, providing valuable references for future studies.
Key Drug
Following the success of PD-(L)1, TIGIT emerged as one of the most promising and prospective targets.
However, its development progress has faced challenges. Taking Roche, a leader in TIGIT research, as an example, in March 2022, Roche announced the results of the Phase III clinical trial SKYSCRAPER-02 for Tiragolumab. This study evaluated the efficacy and safety of Tiragolumab in combination with Tecentriq and chemotherapy as a first-line treatment for extensive-stage small cell lung cancer (ES-SCLC). The results showed that Tiragolumab+Tecentriq+chemotherapy did not meet the co-primary endpoint of progression-free survival (PFS) compared to the control group, and it also did not meet the co-primary endpoint of overall survival (OS) in the subsequent interim analysis. This indicates that Tiragolumab+Tecentriq failed to improve overall survival and control tumor progression.
In May 2022, the SKYSCRAPER-01 study dealt a blow to the development of TIGIT-targeted therapies.
This randomized, double-blind, global multicenter Phase III clinical trial enrolled 534 patients with locally advanced, unresectable, or metastatic non-small cell lung cancer expressing high levels of PD-L1. Patients were randomized in a 1:1 ratio to receive either Tiragolumab+Tecentriq or placebo+Tecentriq.
The results showed that the SKYSCRAPER-01 study did not meet the co-primary endpoint of progression-free survival (PFS), and overall survival (OS) data were immature. However, numerical improvements were observed in both co-primary endpoints. Roche stated that the study would continue to progress until the OS results are obtained.
Roche's setbacks have not dampened the enthusiasm of other pharmaceutical companies. Currently, many companies, including GSK, Novartis, and Gilead, are actively involved in TIGIT research. Some companies are conducting internal research and development, while others are expanding their product lines by introducing TIGIT products.
In June 2021, GSK entered into a collaboration with iTeos to gain joint US rights and exclusive commercialization rights in other regions for iTeos' TIGIT product EOS-448.
EOS-448 is an anti-TIGIT IgG1 monoclonal antibody that binds to TIGIT and blocks its interaction with certain ligands such as CD155 and CD112.
Additionally, it can bind to the ligand CD226 and activate immune responses in T cells and natural killer (NK) cells. iTeos has designed a series of combination studies for EOS-448, including an ongoing Phase Ib clinical trial in combination with GSK's PD-1 inhibitor Jemperli (dostarlimab) for the treatment of non-small cell lung cancer.
Planned Phase Ib clinical trials include combinations of EOS-448, Jemperli, and iTeos' adenosine A2A receptor inhibitor inupadenant, as well as combinations with GSK's anti-CD96 antibody.
In December 2021, Novartis entered into a collaboration with BeiGene for their TIGIT product Ociperlimab. Ociperlimab is an investigational potent TIGIT inhibitor with intact Fc functionality, which is crucial for the anti-tumor activity of TIGIT antibodies.
During the option period, Novartis and BeiGene will collaborate on the clinical development of ociperlimab in combination with tislelizumab. Novartis will design, initiate, conduct, and fund global joint clinical trials.
In addition to their in-house TIGIT monoclonal antibody BMS-986207, BMS is actively searching for potential TIGIT products.
In 2021, BMS entered into a collaboration with Agenus to obtain global exclusive rights to AGEN1777, the latter's proprietary bispecific antibody program targeting TIGIT.
AGEN1777 is designed to block TIGIT and another undisclosed target, and it has been engineered with an enhanced Fc region to improve the activity of T cells and NK cells.
Preclinical studies have demonstrated significant therapeutic potential for AGEN1777 in tumor models where PD-1 monotherapy or TIGIT monotherapy is ineffective.




