Patent Research and Operational Guide for Daiichi Sankyo's ADC Drug DS-8201(1)
Antibody-Drug Conjugates (ADCs) share a universal tripartite structure consisting of an antibody, linker, and payload. Currently, 272 ADC drugs have been initiated for clinical trials globally. Excluding the market withdrawal of Belantamab mafodotin (Blenrep®), 14 approved ADC products remain available. ADCs, with intricate patent positioning strategies, represent a highly competitive field in pharmaceutical research and development. Taking the Daiichi Sankyo's ADC drug DS-8201 as an example, not only do source patents for each component provide initial protection, but the remarkable pharmacodynamic properties of new combinations significantly aid in patent iteration and extension of market exclusivity periods. When developing new drugs from various existing components, it has become essential for companies to comprehensively and accurately extract and summarize source patent information, core patent information, and iterative patent information for each component of the drug under investigation. This process yields high-quality patent research reports, which are crucial for infringement analysis, biosimilar development, and investment and financing activities. The following guide demonstrates how to conduct detailed patent research for ADC drugs.
Disclaimer:
The data presented in this report, primarily sourced from Patsnap Synapse, Bio, Chemical and Analytics databases, may exhibit discrepancies due to data source leakage, variations in statistical periods, and divergent search methodologies. Therefore, it should be regarded as a reference only. This report is not responsible for any business losses resulting from the usage of this information.
Based on the characteristics of ADC drugs, we have summarized the process for conducting patent research on ADC drugs. You can follow the process described below to carry out patent research on ADC drugs.
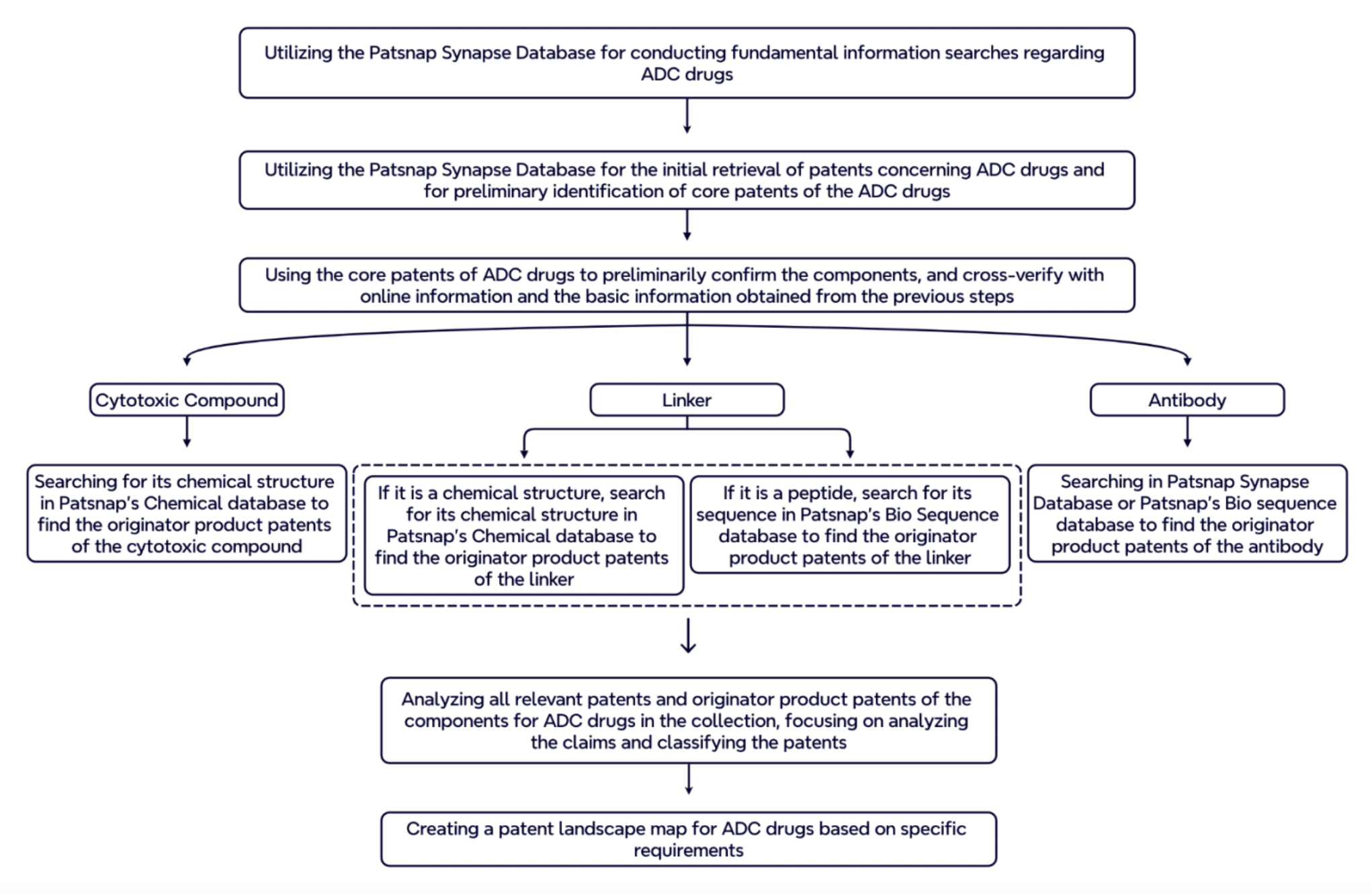
For more information, please click the image link below to access the full report.