Pfizer Announces Positive Phase III Results for ADCETRIS® in Treating Resistant Large B-cell Lymphoma
Pfizer Inc. has declared that their recent Phase 3 clinical trial involving ADCETRIS® (brentuximab vedotin) used in tandem with the medications lenalidomide and rituximab for patients suffering from relapsed or refractory diffuse large B-cell lymphoma has yielded profound and statistically substantial enhancements in patient longevity. This improvement is notably superior when compared to the combination of lenalidomide, rituximab, and a placebo.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The profile of ADCETRIS' safety and tolerability observed in the ECHELON-3 study aligns with previous outcomes noted for patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who were administered ADCETRIS in past clinical research. The comprehensive outcomes are slated to be disclosed during a future healthcare conference.
Roger Dansey, M.D., Pfizer's Chief Development Officer in Oncology, expressed his enthusiasm, "With this being our third Phase 3 research showing a survival advantage for a regimen including ADCETRIS in a certain lymphoma subset, we are hopeful about ADCETRIS' potential to fulfill a significant treatment void for individuals with relapsed or refractory DLBCL, irrespective of CD30 levels."
Dr. Dansey remarked on the promising nature of the findings, emphasizing that the participant pool consisted of patients who had undergone extensive previous treatments, with some having had former CAR-T therapy.
As the predominant lymphoma variant, DLBCL is characterized by rapid progression and is considered a highly aggressive hematological malignancy. Post-initial therapy, around 40% of DLBCL patients either do not achieve a response or encounter disease recurrence.
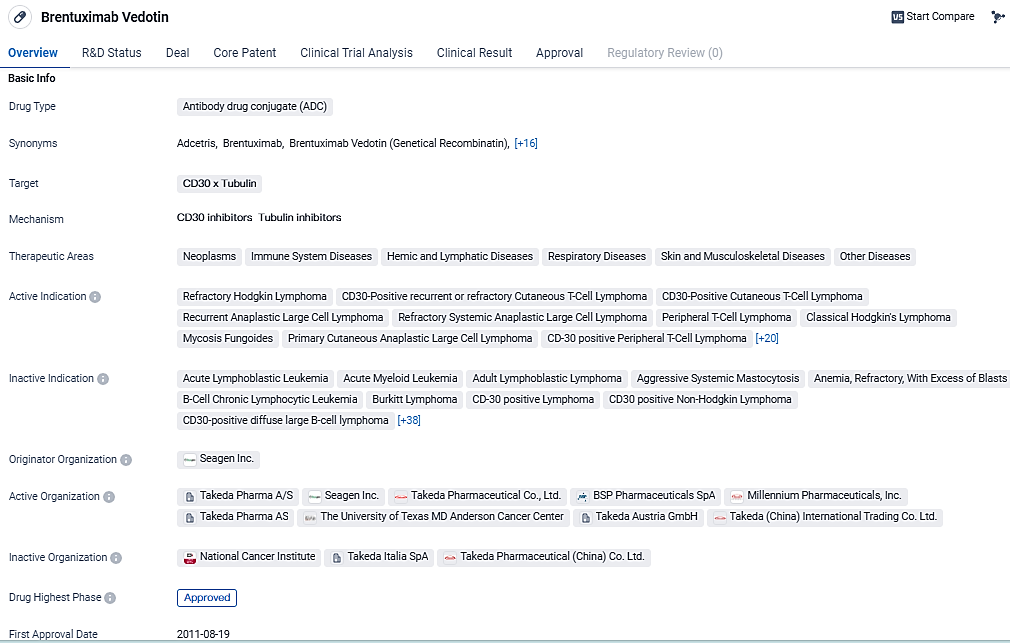
In the realm of lymphoma care, ADCETRIS is established as a therapeutic staple and boasts seven sanctioned uses within the United States, having been administered to upwards of 55,000 individuals since its inaugural U.S. approval over a decade ago in 2011. The global patient reach for ADCETRIS treatment has exceeded 140,000. Following the ECHELON-3 findings, Pfizer intends to engage with the U.S. Food and Drug Administration regarding potential support for a regulatory submission in the United States.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
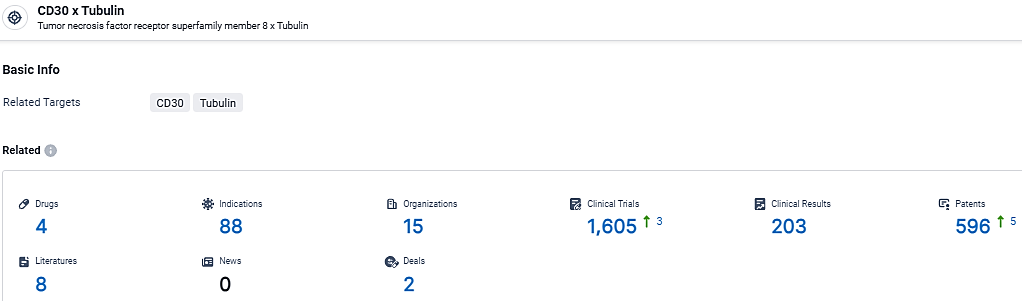
According to the data provided by the Synapse Database, As of March 14, 2024, there are 4 investigational drugs for the CD30 and Tubulin target, including 88 indications, 15 R&D institutions involved, with related clinical trials reaching 1605, and as many as 596 patents.
Brentuximab Vedotin is an ADC drug that targets CD30 and Tubulin. It has been approved for the treatment of numerous neoplasms and other diseases. Its approval in multiple countries, including the United States and China, demonstrates its potential as a valuable therapeutic option in the field of biomedicine.