Phanes Therapeutics reports its first patient has been administered with PT217 in a Phase 1 trial
Phanes Therapeutics, Inc., a biotech firm in the clinical trial phase with its main focus on novel drug discovery and oncology-centric development, made an announcement concerning its Phase 1 clinical trial of PT217. This trial, the first-in-class, involves a native IgG-like bispecific antibody which targets DLL3 and CD47. The first patient in this study has now been treated and the primary objective of this trial is the treatment of small cell lung cancer along with other neuroendocrine tumors.
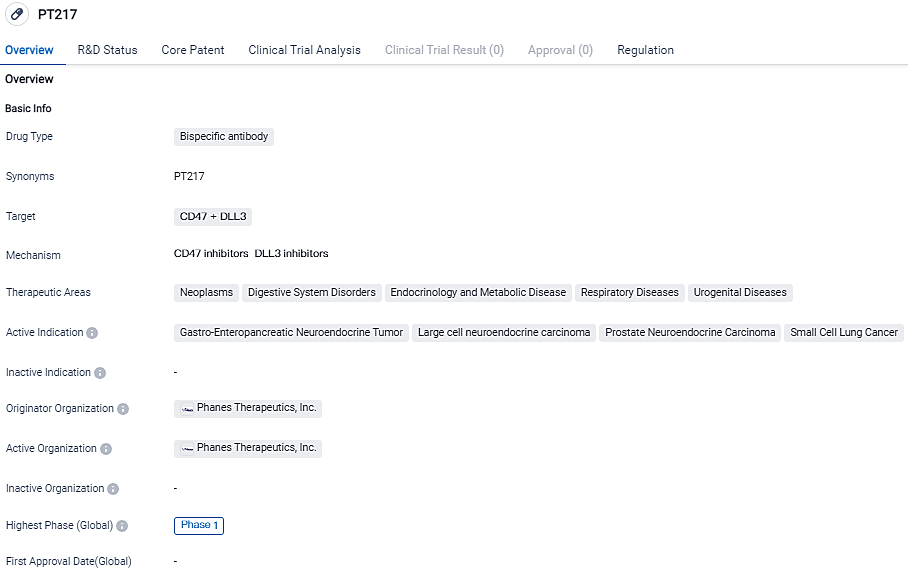
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
PT217, a common light chain bispecific antibody, was discovered using Phanes' research engine and received FDA approval as an orphan drug for treating small cell pulmonary carcinoma last year.
By targeting both DLL3 and CD47 expressed on cancer cell surfaces, PT217 is expected to exterminate tumor cells directly through macrophages' ADCP activity and NK cells' ADCC activity, thereby expanding its tumor killing spectrum. Moreover, PT217 could potentially instigate tumor neoantigen presentation by directing tumor cells towards phagocytotic antigen-presenting cells and indirectly spur T-cell killing of DLL3-expressing tumor cells through tumor neoantigen recognition, thus activating the adaptive immune response.
The anti-CD47 arm of PT217 is differentiated, showing little linkage to human erythrocytes but maintaining a strong affinity for CD47 on cancer cells. "Small cell lung cancer is among the most destructive and aggressive solid tumors, leaving patients without effective treatment options. PT217, born from Phanes' dynamic innovation in generating new therapeutic strategies and practical technologies, may provide a new alternative for these patients," commented Dr. Ming Wang, Phanes' Founder and Chief Executive.
PT217's multi-center Phase I clinical trial is assessing its safety, tolerability, pharmacokinetics, pharmacodynamics, and early efficacy in patients suffering from inoperable or small cell lung cancer, large cell neuroendocrine cancer, neuroendocrine prostate cancer, and gastroenteropancreatic neuroendocrine carcinoma.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 12, 2023, there are 1 investigational drugs for the CD47 and DLL3 target, including 4 applicable indications,1 R&D institutions involved, with related clinical trials reaching 1,and as many as 929 patents.
Novartis AG is the company with the highest number of drugs in the approved phase under the Gastro-Enteropancreatic Neuroendocrine Tumor. The targets for these drugs include SSTR, SSTR2, and DNA. This analysis provides insights into the current competitive landscape and future development of the GEP-NET indication in the pharmaceutical industry.