Pharmaceutical Insights: Vorinostat's R&D Progress and its Mechanism of Action on Drug Target
Vorinostat's R&D Progress
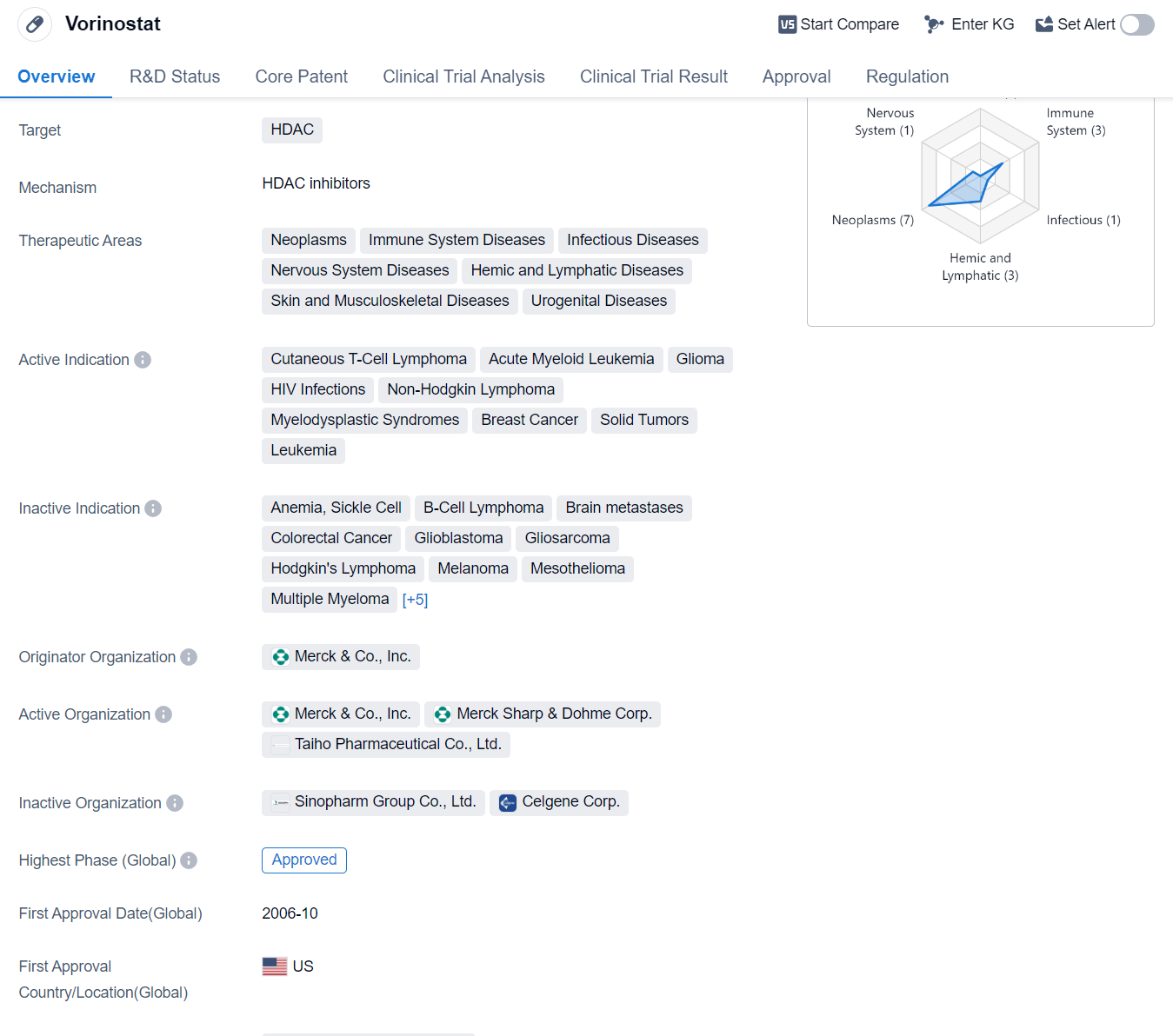
Vorinostat is a small molecule drug that falls under the category of histone deacetylase (HDAC) inhibitors. It is primarily used in the treatment of various diseases related to the immune system, neoplasms, infectious diseases, nervous system diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. Some specific indications for Vorinostat include cutaneous T-cell lymphoma, acute myeloid leukemia, glioma, HIV infections, non-Hodgkin lymphoma, myelodysplastic syndromes, breast cancer, solid tumors, and leukemia.
The drug was originally developed by Merck & Co., Inc. and received its first approval in the United States in October 2006. Vorinostat has been granted orphan drug status, which indicates that it is intended for the treatment of rare diseases or conditions.
Vorinostat has reached the highest phase of development which is approved globally.However, in China, the drug's highest phase is listed as discontinued, suggesting that it may not have met the necessary requirements for approval in that country.
Vorinostat's mechanism of action involves inhibiting HDAC enzymes, which play a role in the regulation of gene expression. By inhibiting these enzymes, Vorinostat can potentially alter gene expression patterns and affect cellular processes, leading to therapeutic benefits in the treatment of various diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Vorinostat: HDAC inhibitor
HDAC inhibitors are a type of drugs that target and inhibit the activity of histone deacetylases (HDACs). HDACs are enzymes that play a crucial role in the regulation of gene expression by removing acetyl groups from histone proteins. By inhibiting HDACs, HDAC inhibitors can increase the acetylation of histones, leading to changes in chromatin structure and gene expression.
From a biomedical perspective, HDAC inhibitors have been extensively studied for their potential therapeutic applications in various diseases, including cancer, neurodegenerative disorders, and inflammatory conditions. In cancer treatment, HDAC inhibitors have shown promise in reversing the aberrant epigenetic modifications that contribute to tumor growth and progression. They can induce cell cycle arrest, promote apoptosis (programmed cell death), inhibit angiogenesis (formation of new blood vessels), and enhance the immune response against cancer cells.
Furthermore, HDAC inhibitors have been investigated for their neuroprotective effects in neurodegenerative diseases like Alzheimer's and Parkinson's. By modulating gene expression and promoting neuronal survival, these inhibitors have the potential to slow down disease progression and alleviate symptoms.
In the context of inflammatory conditions, HDAC inhibitors have been shown to suppress the production of pro-inflammatory cytokines and promote anti-inflammatory responses. This makes them a potential therapeutic option for diseases such as rheumatoid arthritis and inflammatory bowel disease.
It is important to note that HDAC inhibitors can have side effects, including gastrointestinal disturbances, fatigue, and hematological abnormalities. Additionally, their efficacy and safety profiles may vary depending on the specific inhibitor and the disease being targeted. Therefore, further research and clinical trials are needed to fully understand the potential of HDAC inhibitors as therapeutic agents.
Drug Target R&D Trends for Vorinostat
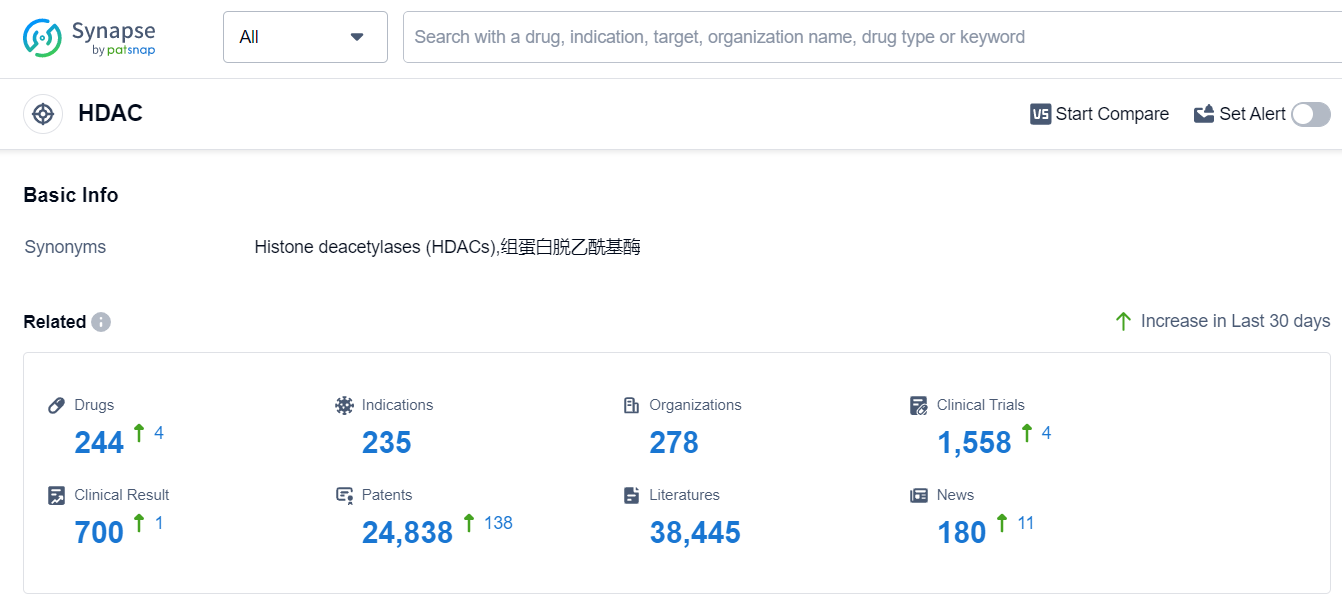
The analysis of the current competitive landscape and future development of target HDAC reveals several key insights. Bristol Myers Squibb Co., Boao Biology Group Co. Ltd., Amylyx Pharmaceuticals, Inc., Great Novel Therapeutics Biotech & Medicals Corp., Merck & Co., Inc., Novartis AG, Aurobindo Pharma Ltd., Acer Therapeutics, Inc., Secura Bio, Inc., Otsuka Holdings Co., Ltd., Orpharma Pty Ltd., Horizon Therapeutics Plc, Eurocept International B.V., JX Nippon Mining & Metals Corp., OrphanPacific, Inc., Immedica Pharma AB, Meiji Holdings Co., Ltd., Groupe Pharmaceutique Boivin, Inc., Syndax Pharmaceuticals, Inc., and Spectrum Pharmaceuticals, Inc. are the companies growing fastest under the current target HDAC. The highest stage of development on this target is the "Approved" phase, indicating regulatory approval for certain drugs. The indications for these drugs vary, including Peripheral T-Cell Lymphoma, Cutaneous T-Cell Lymphoma, Breast Cancer, Multiple Myeloma, Amyotrophic Lateral Sclerosis, and others. Small molecule drugs, Synthetic peptides, Cytokines, and Unknown drugs are progressing most rapidly under the current targets. The United States, Japan, Canada, European Union, and China are developing fastest under the current targets, with China showing notable progress. Overall, the target HDAC presents a competitive landscape with diverse companies, indications, drug types, and global development. Further research and analysis are recommended to gain deeper insights into specific companies, drugs, and countries/locations for strategic decision-making in the pharmaceutical industry.
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 244 HDAC drugs worldwide, from 278 organizations, covering 235 indications, and conducting 1558 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Vorinostat is a small molecule drug that has been approved for use in the United States and has shown potential in the treatment of multiple diseases, particularly those related to the immune system and neoplasms. Its orphan drug status highlights its potential for addressing rare diseases or conditions. However, its discontinuation in China suggests that further research and development may be required to gain approval in that market.