Phase 2a Trial: Endeavor BioMedicines’ ENV-101 Improves Lung Function in Idiopathic Pulmonary Fibrosis Patients
Endeavor BioMedicines, a clinical-stage biotechnology firm focused on creating treatments aimed at providing significant clinical advantages to patients with severe diseases, reported findings from a finished Phase 2a clinical trial. The results indicate that their main investigational drug, ENV-101, enhanced lung function and reversed key indicators of lung fibrosis in idiopathic pulmonary fibrosis patients within a 12-week timeframe.
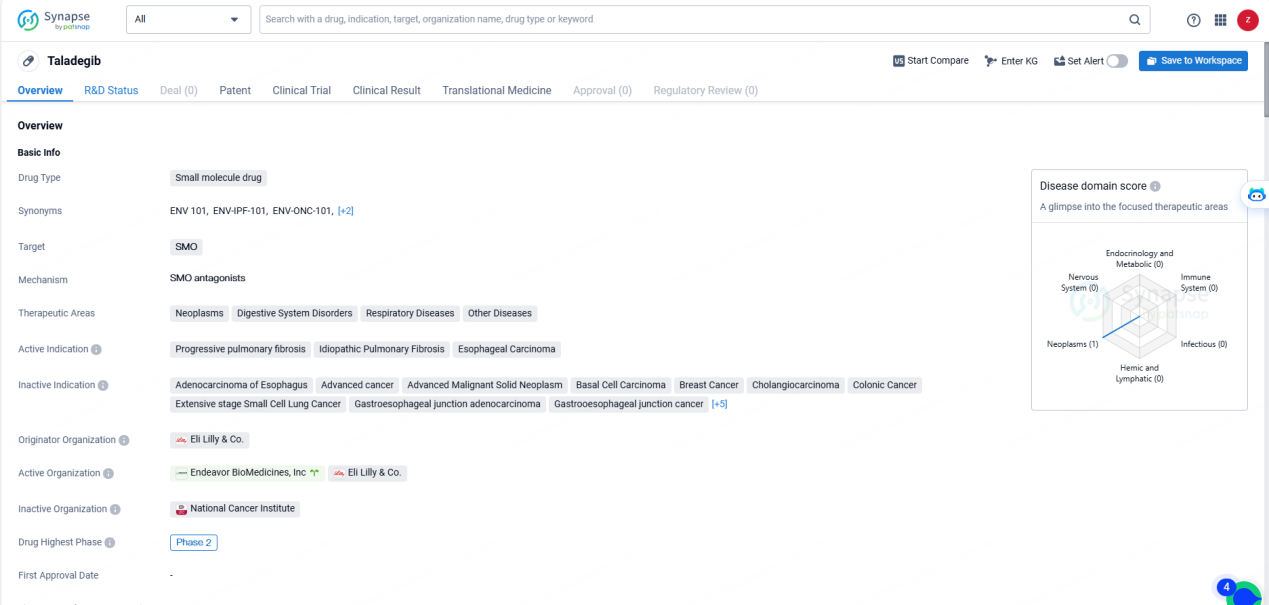
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The findings, which demonstrated that ENV-101 has an acceptable tolerability profile, were unveiled today for the first time in a late-breaking oral session at the American Thoracic Society 2024 International Conference. "There is an urgent need for IPF therapies that offer more than just a slowed decline in lung function," stated Toby M. Maher, M.D., Ph.D., Professor of Medicine and Director of Interstitial Lung Disease at Keck School of Medicine, University of Southern California, Los Angeles, who presented the data. "The early indications of clinical and antifibrotic activity suggest that ENV-101 has the potential to alter the course of this relentless disease."
Currently, standard-of-care therapies do not target the root cause of IPF. While they slow the deterioration of lung function, they do not halt or reverse it and have tolerability concerns that restrict their long-term use in most patients. ENV-101 is designed to inhibit a cellular wound-healing mechanism known as Hedgehog that is aberrantly activated in fibrotic lung diseases like IPF, leading to the continuous pathological accumulation of scar tissue in the lungs.
Patients who received ENV-101 showed a statistically significant improvement in lung function over the 12-week trial period, with an average enhancement of 1.9% in percent predicted forced vital capacity from baseline, compared to an average decline of 1.3% in the placebo group.
Patients treated with ENV-101 exhibited a statistically significant increase in total lung capacity (TLC) above baseline at 12 weeks, with a mean rise of 200 mL compared to a mean reduction of 56 mL in the placebo group (P=0.005). Among those treated with ENV-101, 80% experienced an increase in TLC, whereas 70% of the placebo group showed a decrease in TLC.
"These trial outcomes suggest that ENV-101 could offer significant clinical benefits for individuals with IPF, who are in need of therapies that can shift treatment goals from merely slowing disease progression to potentially reversing it," said Paul A. Frohna, M.D., Ph.D., Chief Medical Officer at Endeavor BioMedicines. "We are eager to further explore ENV-101's potential in the upcoming WHISTLE-PF clinical trial."
Based on the results from the ENV-101 Phase 2a trial presented today, Endeavor BioMedicines plans to launch the Phase 2 WHISTLE-PF (Wound-remodeling Hedgehog-inhibitor ILD Study Testing Lung Function Endpoints -PF) trial. This trial is anticipated to include a global Phase 2b cohort for individuals with IPF and a parallel Phase 2 cohort for individuals with progressive pulmonary fibrosis.
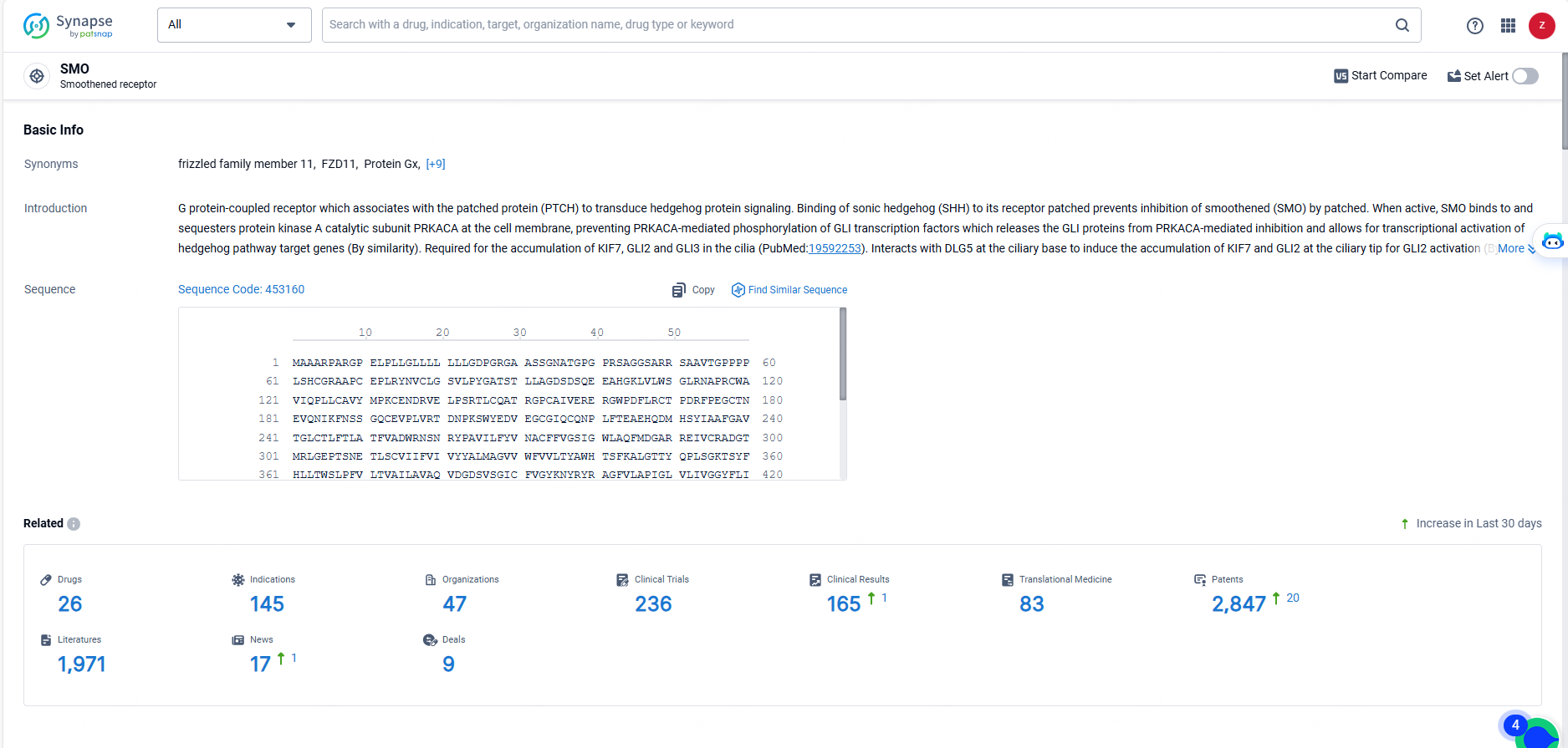
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 26 investigational drugs for the SMO targets, including 145 indications, 47 R&D institutions involved, with related clinical trials reaching 236, and as many as 2847 patents.
ENV-101 is a small molecule drug that targets the Smoothened (SMO) protein. It is being developed for the treatment of various therapeutic areas including neoplasms, digestive system disorders, respiratory diseases, and other diseases. The active indications for ENV-101 include progressive pulmonary fibrosis, idiopathic pulmonary fibrosis, and esophageal carcinoma.