Phase 3 FLOW Study: 1mg Ozempic® Reduces Kidney Disease Risk
At the 84th Annual Scientific Sessions of the American Diabetes Association, Novo Nordisk unveiled the comprehensive findings from the FLOW trial, a phase 3b study that examined the impact of a once-weekly 1 mg injection of semaglutide on adults suffering from type 2 diabetes and chronic kidney disease.
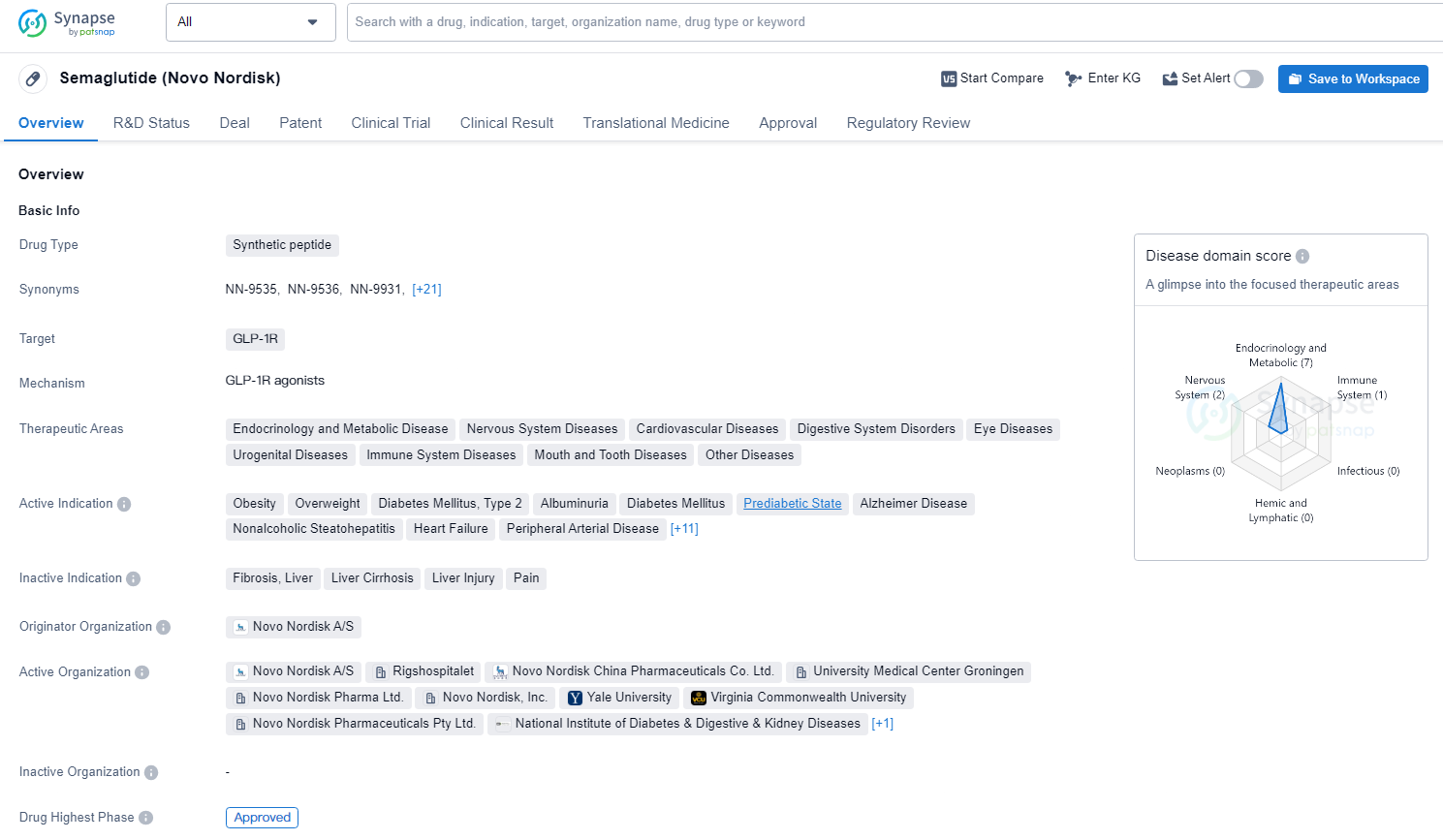
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The double-blind, randomized, placebo-controlled study involving 3,533 participants with type 2 diabetes and chronic kidney disease (CKD) reached its main objective. Semaglutide 1 mg led to a 24% decrease in the risk of kidney disease progression and mortality related to cardiovascular and kidney conditions when compared to placebo. The primary endpoint included major kidney disease events, defined as the onset of kidney failure, a persistent 50% or more reduction in eGFR from baseline, or death due to kidney-related or cardiovascular origins.
“Addressing severe comorbid conditions like chronic kidney disease is crucial for improving treatment outcomes in type 2 diabetes patients. The FLOW trial results provide significant insights as we seek to understand the potential benefits of GLP-1 treatments for this patient group,” stated Anna Windle, PhD, senior vice president of clinical development, Medical & Regulatory Affairs at Novo Nordisk.
The FLOW trial was terminated early by Novo Nordisk following a recommendation from an Independent Data Monitoring Committee, due to meeting pre-specified efficacy outcomes after a median follow-up of 3.4 years.
Based on outcomes from the FLOW study, Novo Nordisk has applied for a label extension for Ozempic, which has been accepted for review by the U.S. Food & Drug Administration, with a decision anticipated in January 2025. The data from the FLOW study were previously disclosed in May at the 61st European Renal Association Congress and concurrently published in the New England Journal of Medicine.
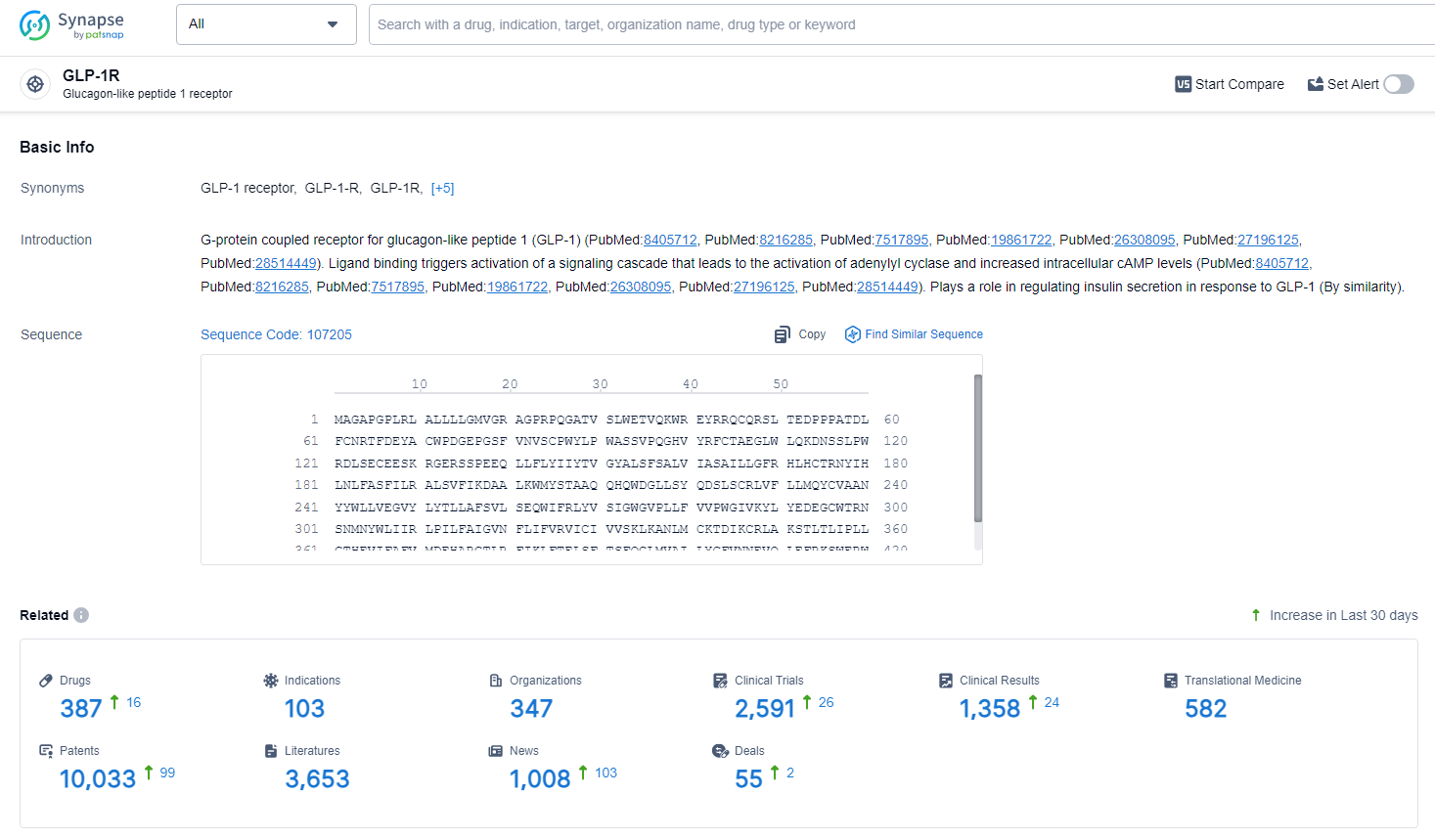
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 28, 2024, there are 387 investigational drugs for the GLP-1R target, including 103 indications, 347 R&D institutions involved, with related clinical trials reaching 2591, and as many as 10033 patents.
As a synthetic peptide targeting GLP-1R, Semaglutide represents a significant advancement in the pharmaceutical industry, particularly in the treatment of a wide range of diseases and conditions. Its approval in multiple therapeutic areas and its breakthrough designation underscore its potential to make a meaningful impact on patient care and public health. Semaglutide's approval and regulatory status, along with its broad therapeutic potential, position it as a valuable asset in the pharmaceutical industry, with the potential to address a wide range of unmet medical needs and improve patient outcomes.