Pliant Therapeutics Reports Favorable Phase 2a Trial Outcomes for Bexotegrast in Liver Fibrosis Suspected Cases
Pliant Therapeutics, Inc. has unveiled partial data for the 12-week mark from the group receiving a 320 mg dosage in the INTEGRIS-PSC study. This international, multicentric Phase 2a study, which is conducted in a randomized, double-blinded, and placebo-controlled manner, is examining the impact of bexotegrast on individuals who are believed to have moderate to severe liver scarring, a condition known as primary sclerosing cholangitis.
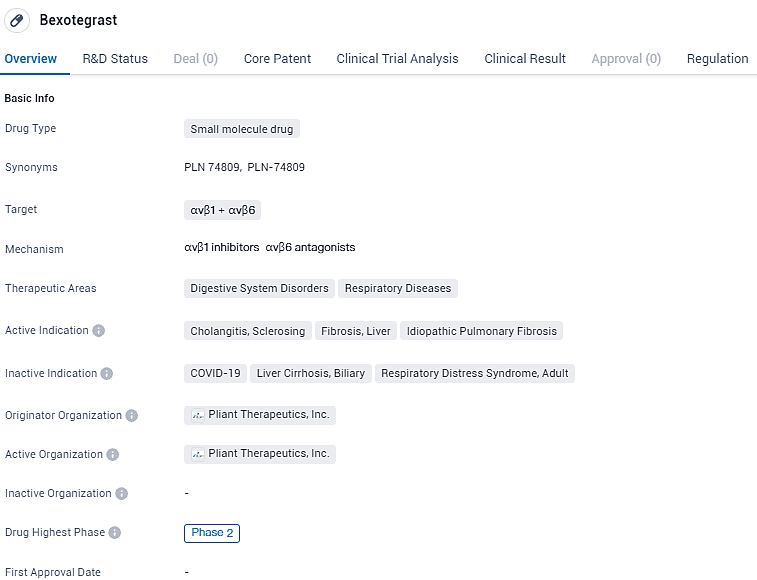
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The group receiving a dosage of 320 mg achieved both its main objectives as well as additional goals, indicating that bexotegrast is generally well-received throughout a treatment span of twelve weeks. Also, a rise in the drug concentration in the plasma corresponded with an increase in dosage. Nonetheless, there was no apparent correlation between dosage and the occurrence of any negative side effects. Incidences of itching and cholangitis were notably less common with the administration of bexotegrast compared to a placebo.
In this trial, investigative endpoints sought to examine alterations in biomarkers for liver fibrosis, namely the Enhanced Liver Fibrosis (ELF) score and PRO-C3 levels. Other aspects under scrutiny included the liver's biochemical health and its visualization through magnetic resonance imaging (MRI). Corroborating former findings from lesser dosages, participants treated with bexotegrast at the 320 mg dosage exhibited diminished ELF scores and PRO-C3 levels in comparison to the placebo group after 12 weeks.
Patients treated with bexotegrast equally displayed stable levels of alkaline phosphatase, in contrast to the rise noted in the placebo group at the same 12-week mark. Furthermore, MRI scans provided ongoing evidence of enhancement in hepatocyte functionality and bile circulation with the administration of bexotegrast at 320 mg as opposed to the placebo.
Dr. Éric Lefebvre, the Chief Medical Officer of Pliant, remarked, “The emerging data from the INTEGRIS-PSC study strengthens the already positive safety and tolerability profile of bexotegrast, an aspect of significant relevance in the context of susceptible patient demographics and the protracted nature of treatment regimens.”
Dr. Lefebvre continued, “The characteristics of bexotegrast as a therapeutic agent are becoming increasingly clear with these insights, and it is promising to observe the drug's effects across a range of measures. This points to its prospective value in the management of PSC where there is a dire demand for effective treatments. I am keenly anticipating further updates from the ongoing study by mid-2024 and the ensuing dialogues with regulatory agencies about the potential strategies moving forward.”
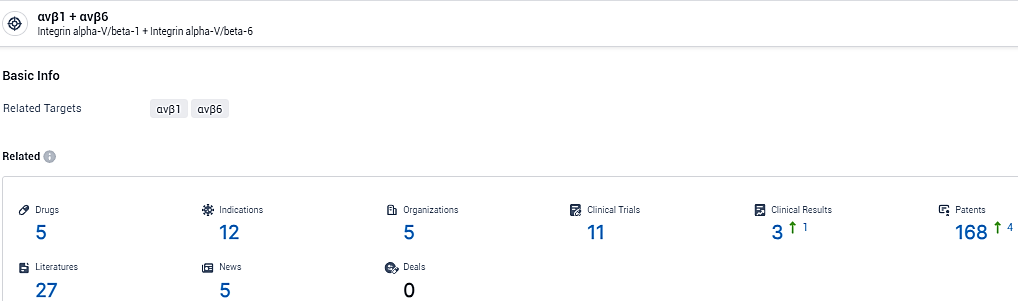
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 8, 2024, there are 5 investigational drugs for the αvβ1 and αvβ6 target, including 12 indications, 5 R&D institutions involved, with related clinical trials reaching 11, and as many as 168 patents.
Bexotegrast targets the αvβ1 and αvβ6 receptors and is indicated for digestive system disorders and respiratory diseases. The drug is currently in Phase 2 of clinical development and has been granted Fast Track and Orphan Drug designations. These regulatory designations highlight the potential of Bexotegrast to address unmet medical needs and provide benefits to patients with rare diseases.