Preview of Clinical Results from ESMO 2024!
On September 9, 2024, the official website of the European Society for Medical Oncology (ESMO) Annual Meeting made public a batch of clinical trial results summaries. The Synapse database immediately launched a search portal for ESMO clinical results data, facilitating user access.
On the results listing page, you can clearly see each ESMO clinical trial entry, including: ID number, title, institution, clinical registration number, clinical phase, population characteristics, clinical trial protocols, clinical results data, and clinical evaluations.
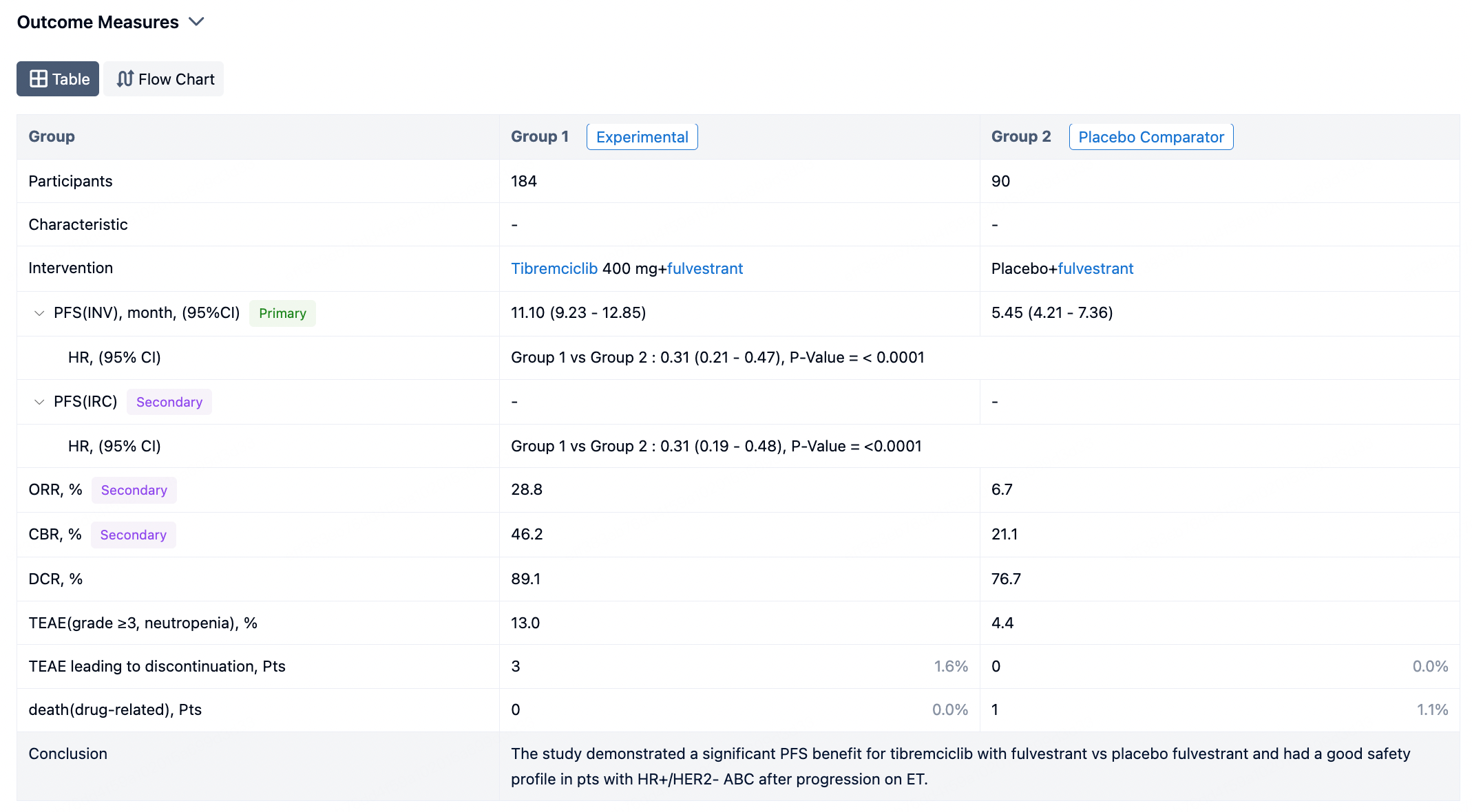
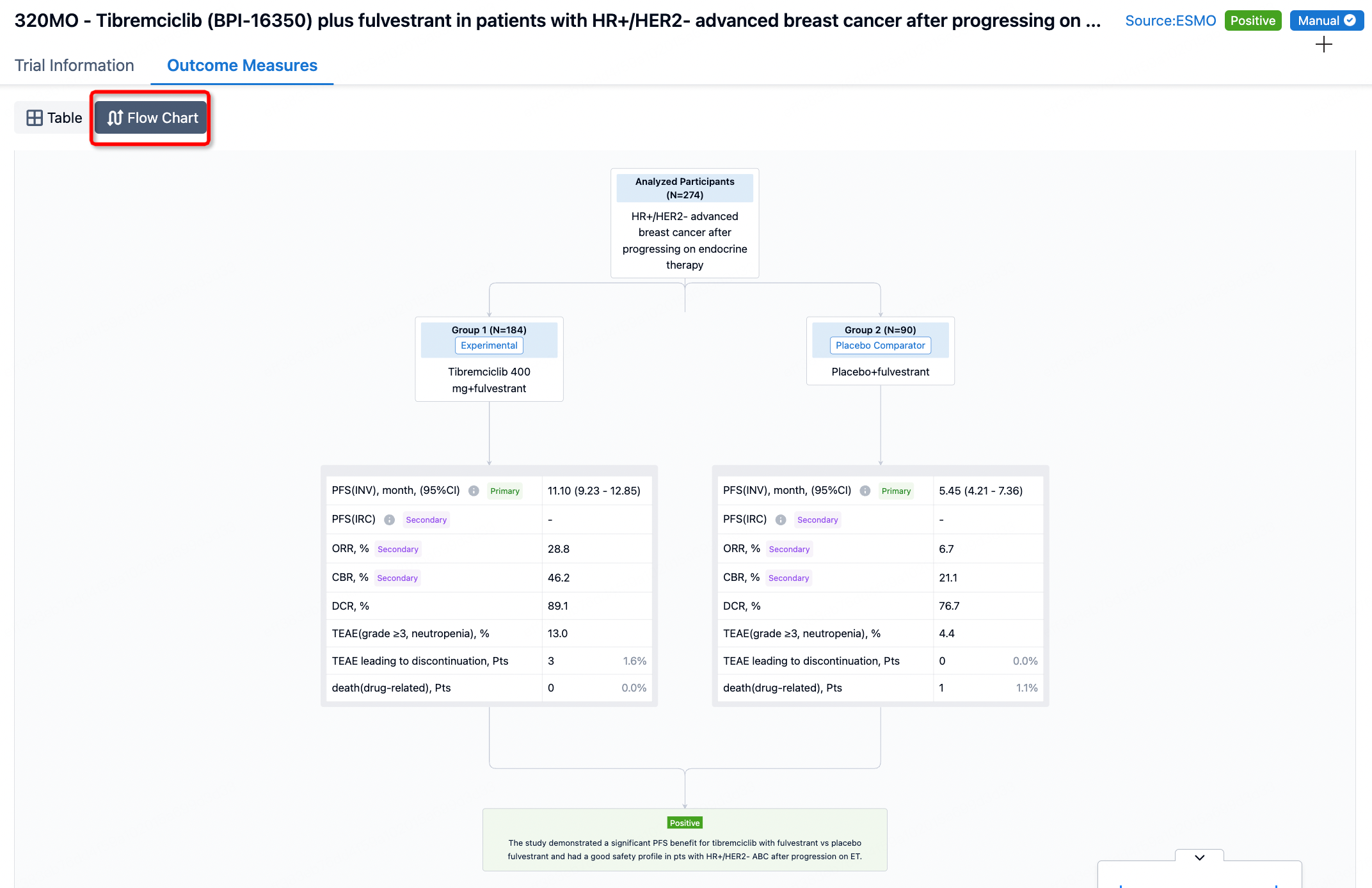
By clicking on a specific clinical trial, you enter a detailed page where you can view comprehensive trial information, as well as endpoint indicator information. The presentation of endpoint indicators is available in two formats: tabular display and flowchart display.
Obtain the ESMO clinical outcomes you want through screening
By searching for Sacituzumab Govitecan + ESMO 2024, you can obtain 2 clinical trial results.
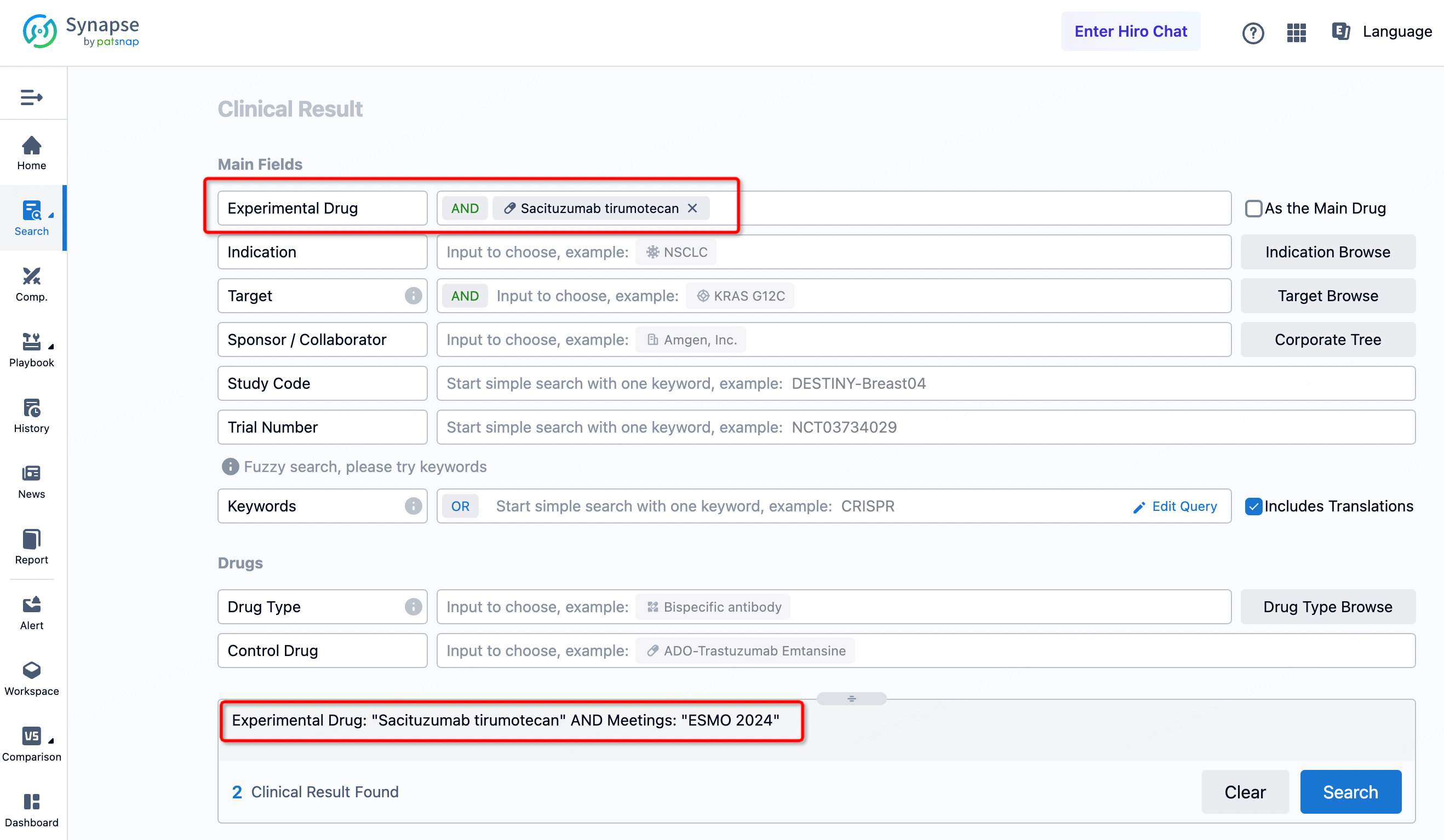
By searching for NSCLC + ESMO 2024, you can obtain 84 clinical trial results.
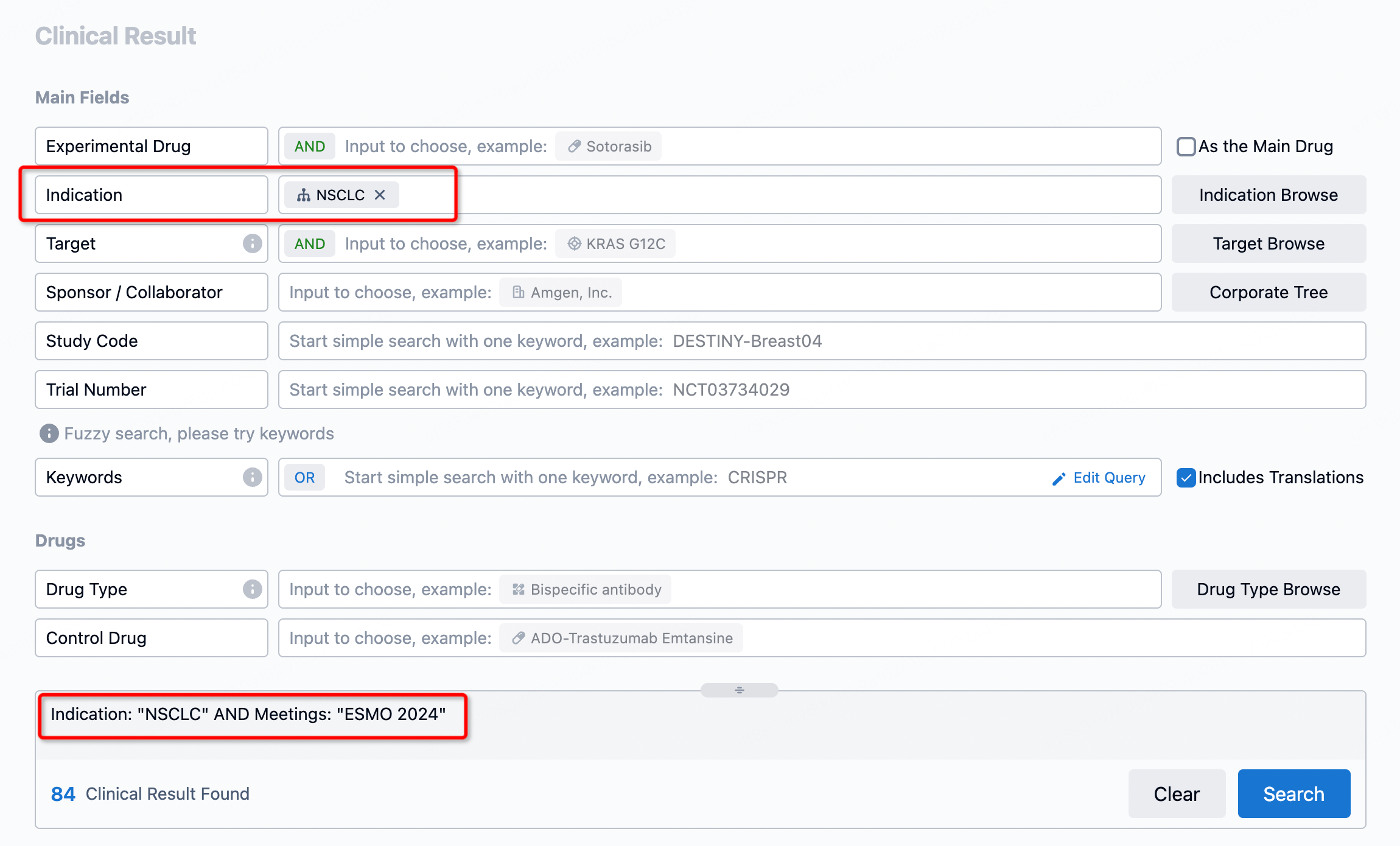
You can obtain precise clinical outcomes by setting up more advanced filtering combinations based on your focus areas.
How to Easily View the Clinical Results Using Synapse Database?
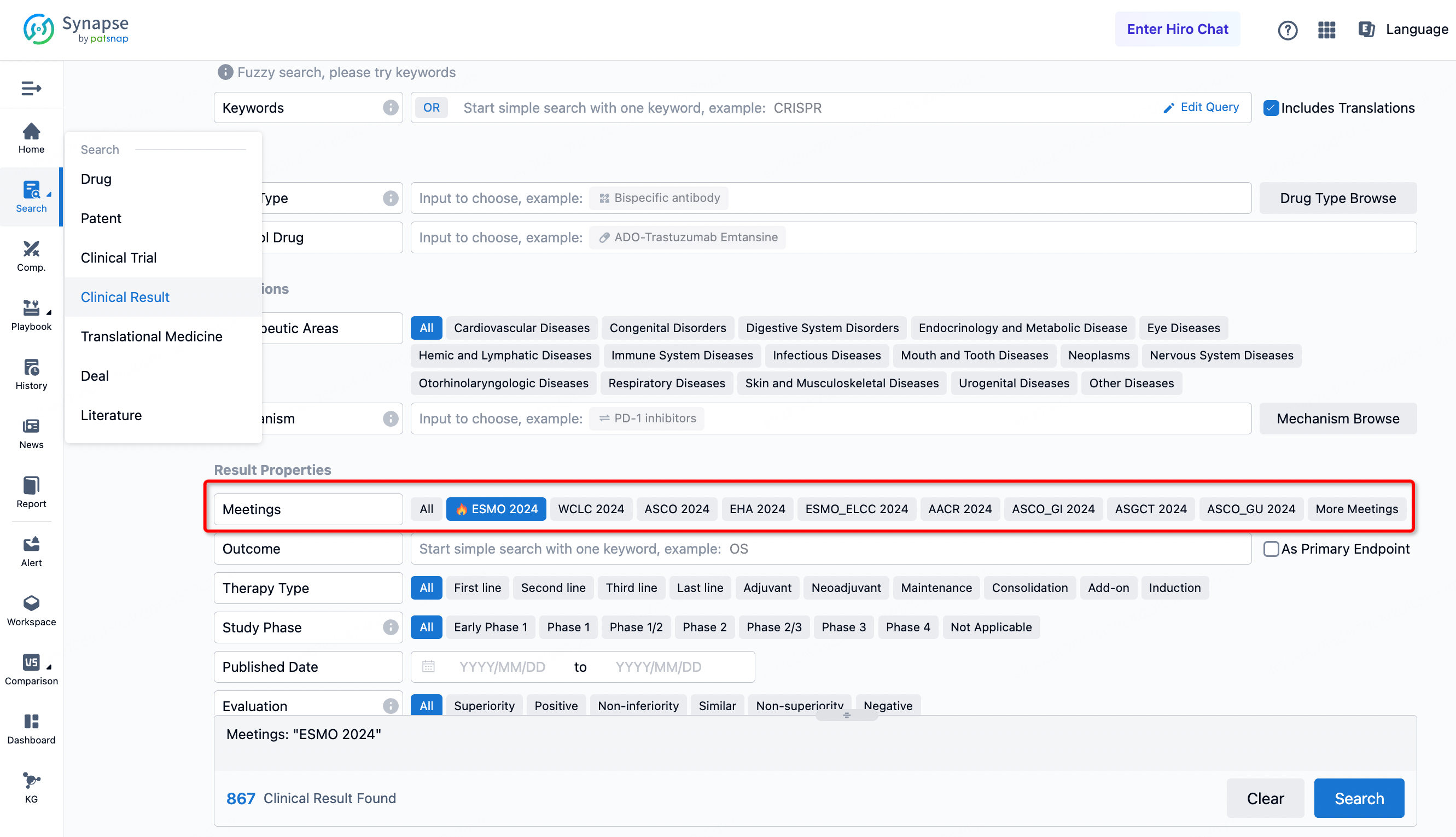
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
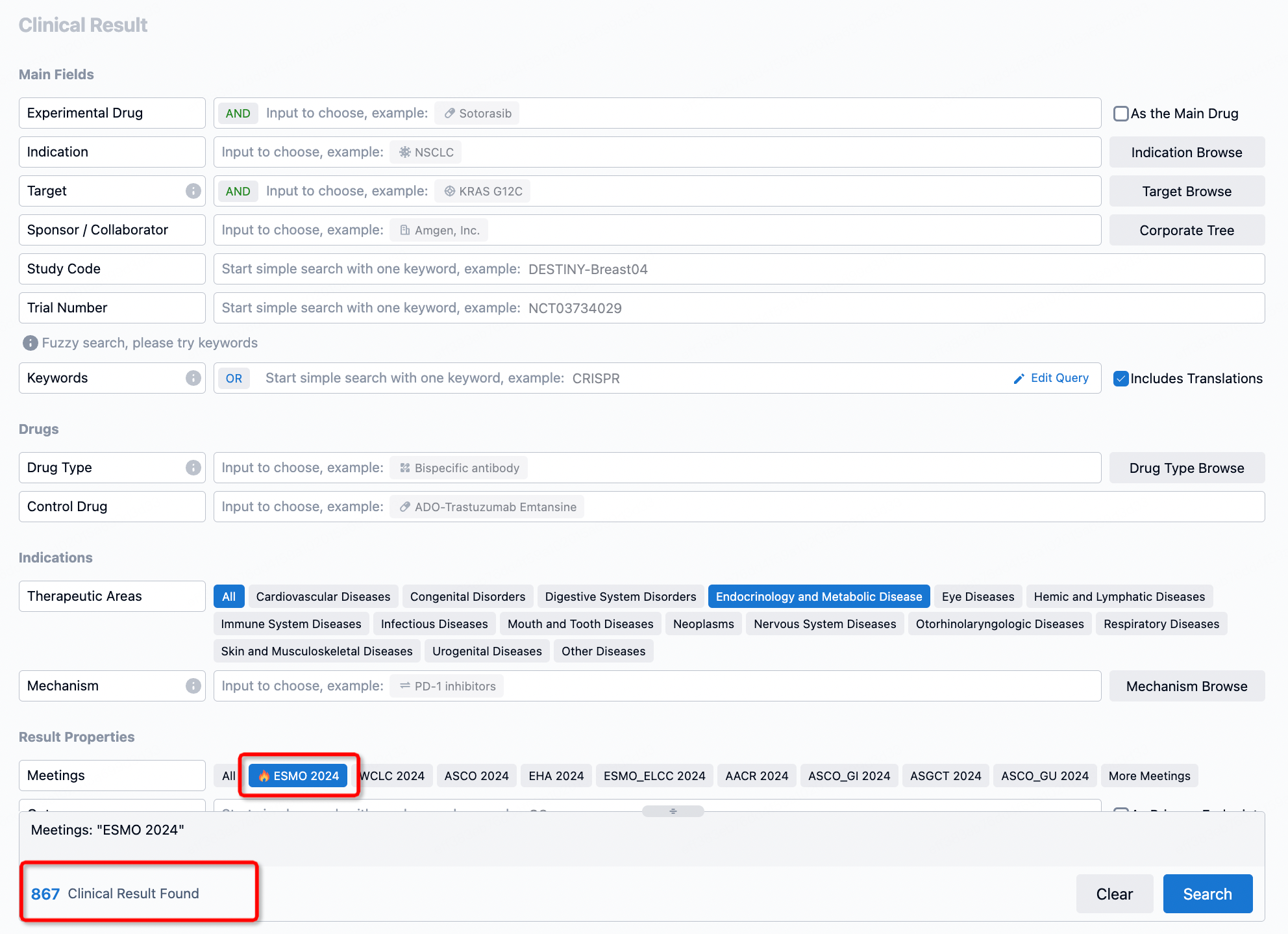
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
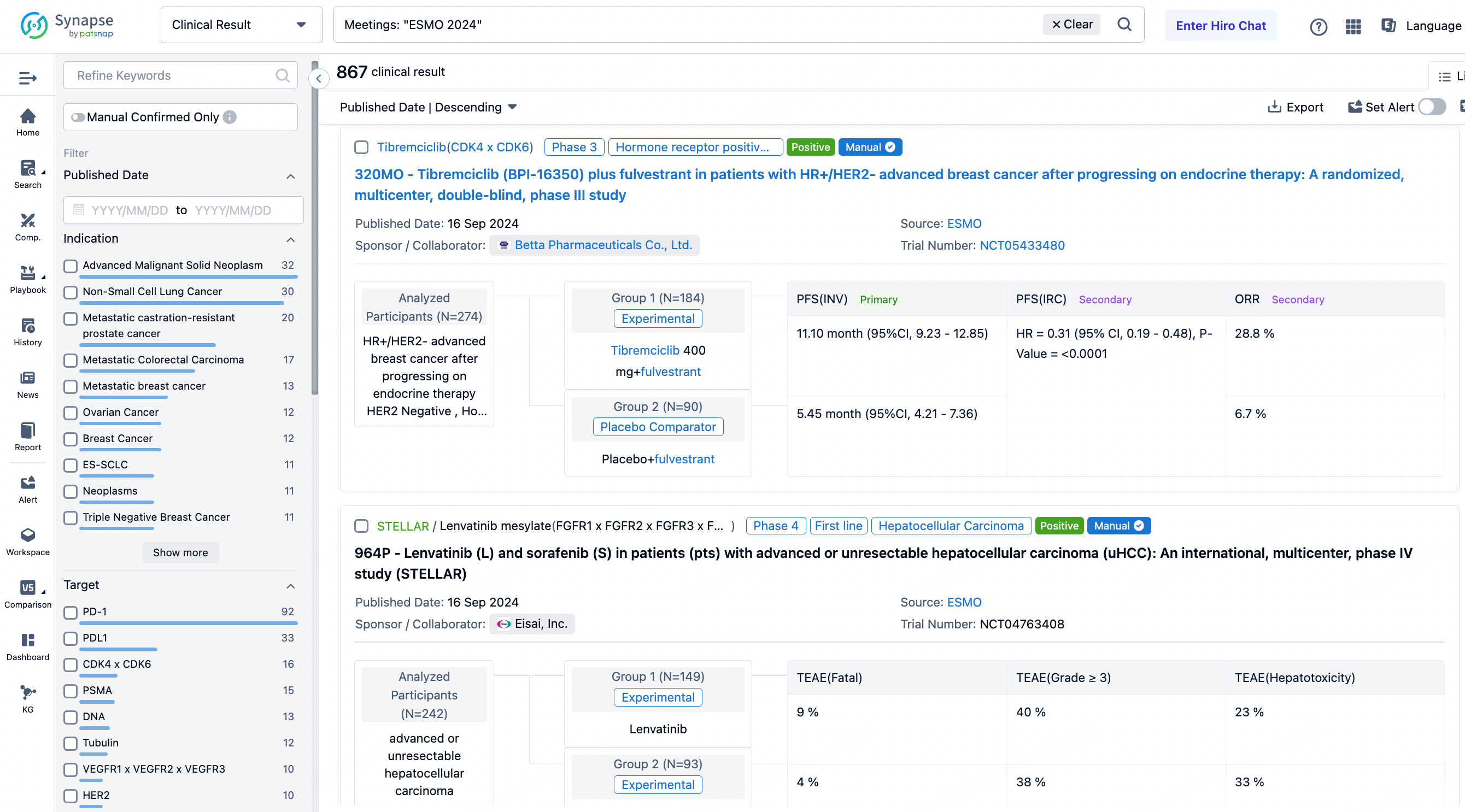
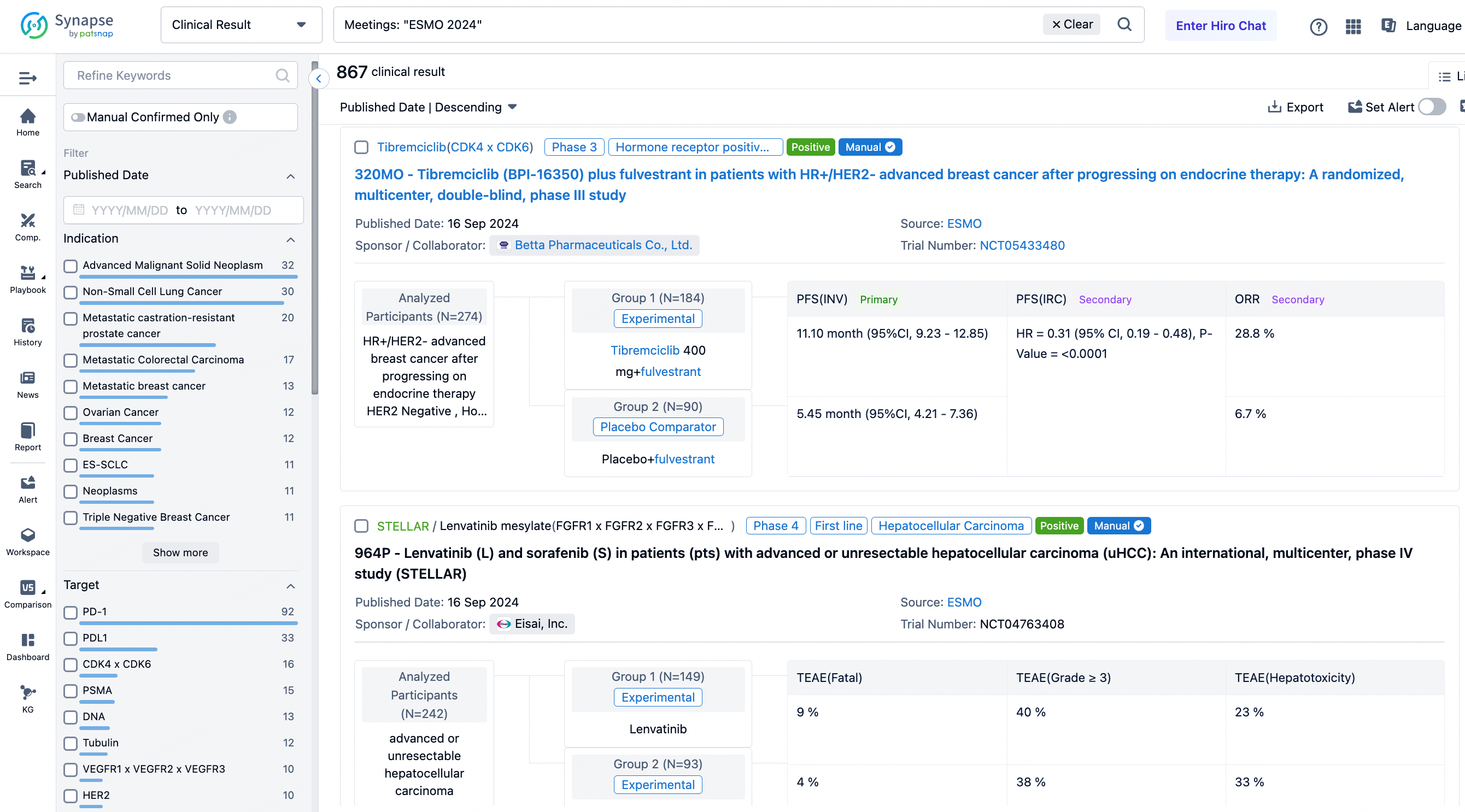
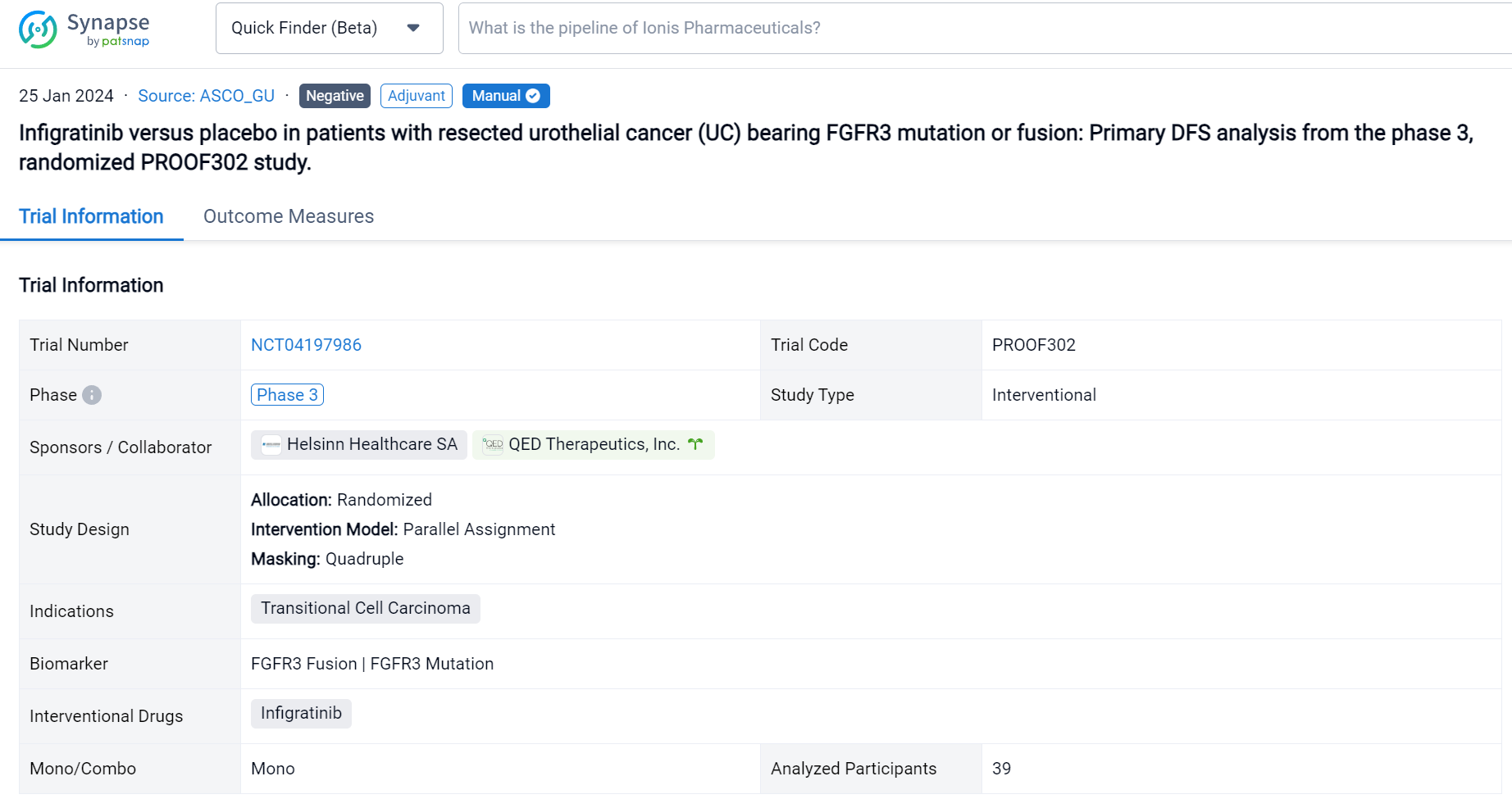
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
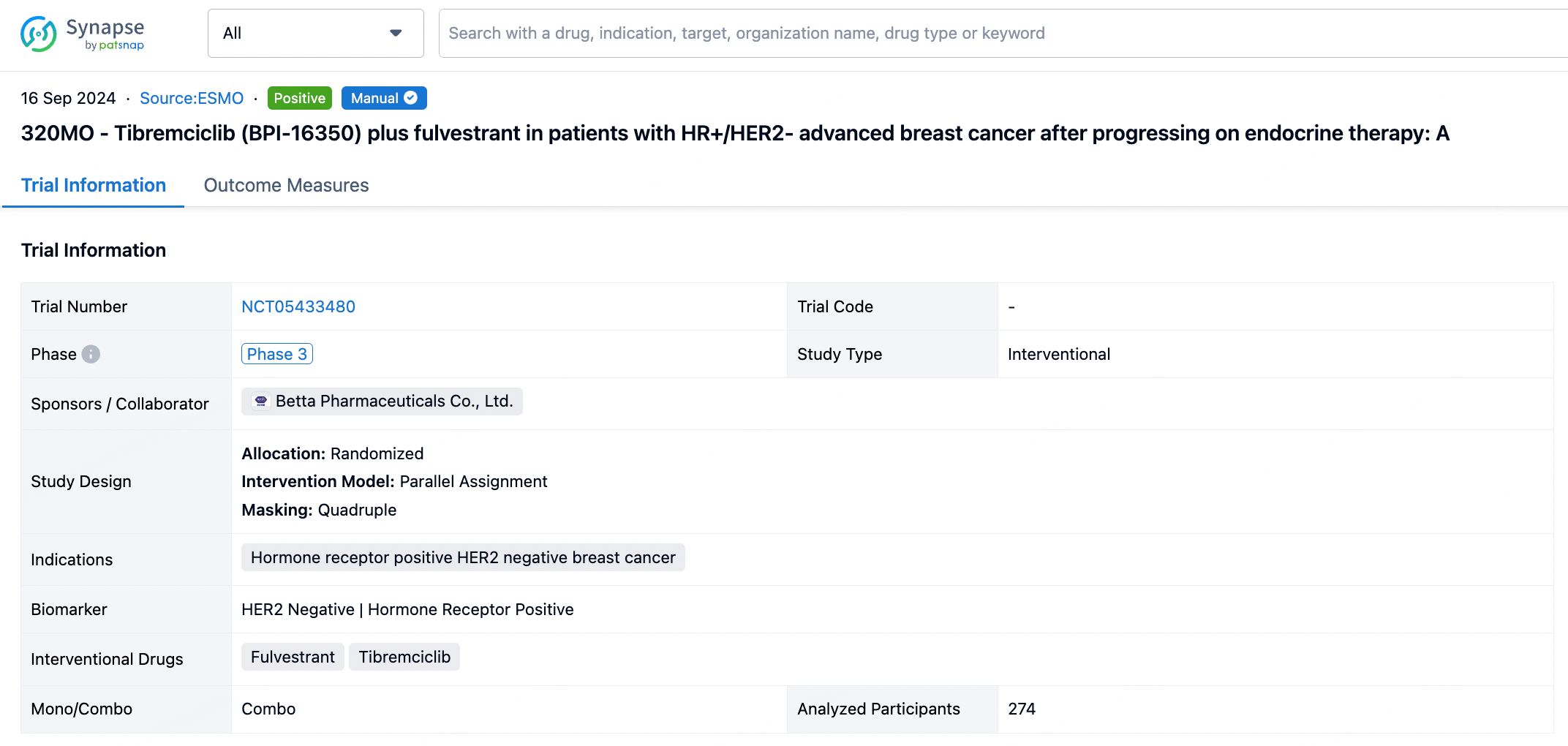
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
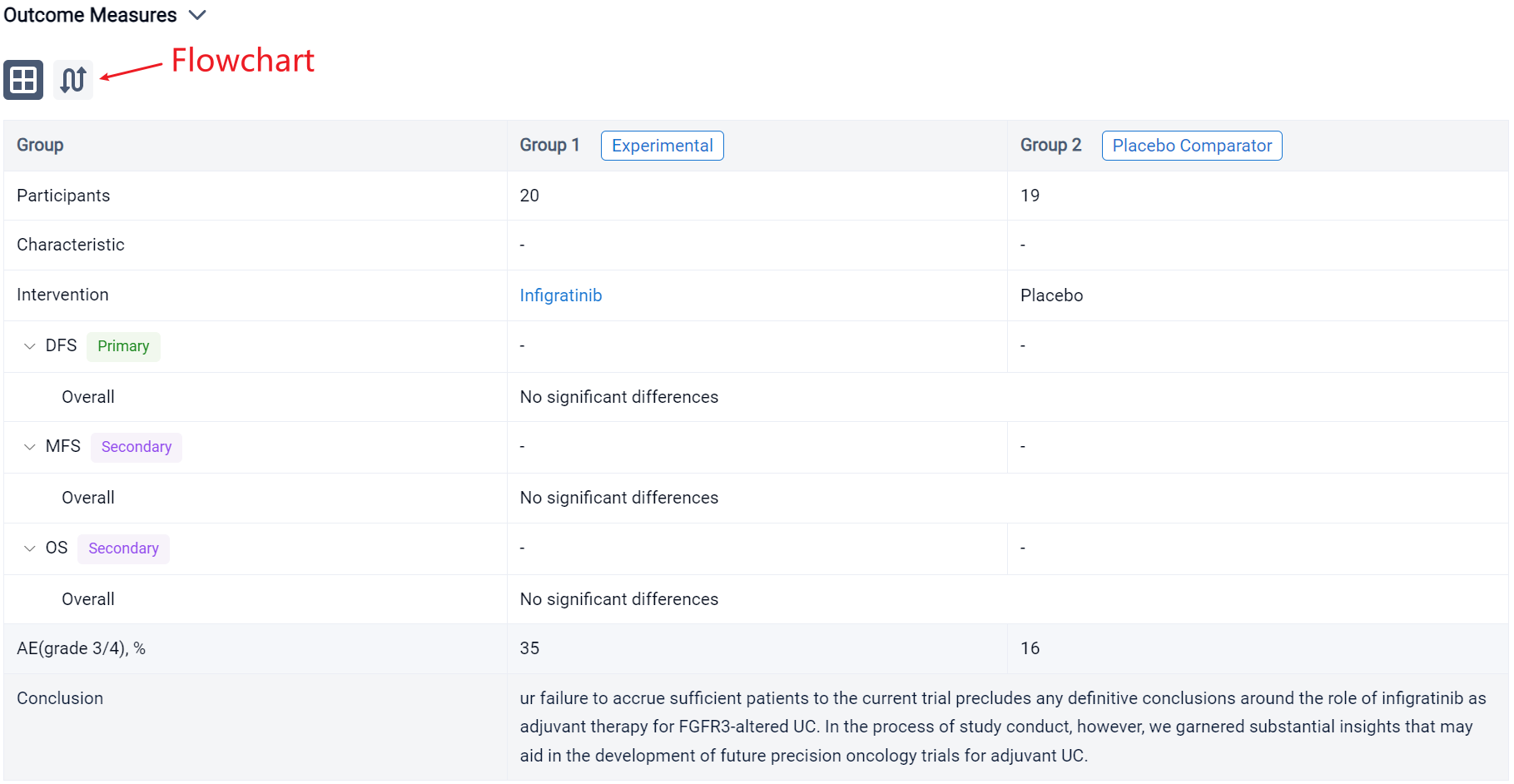
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
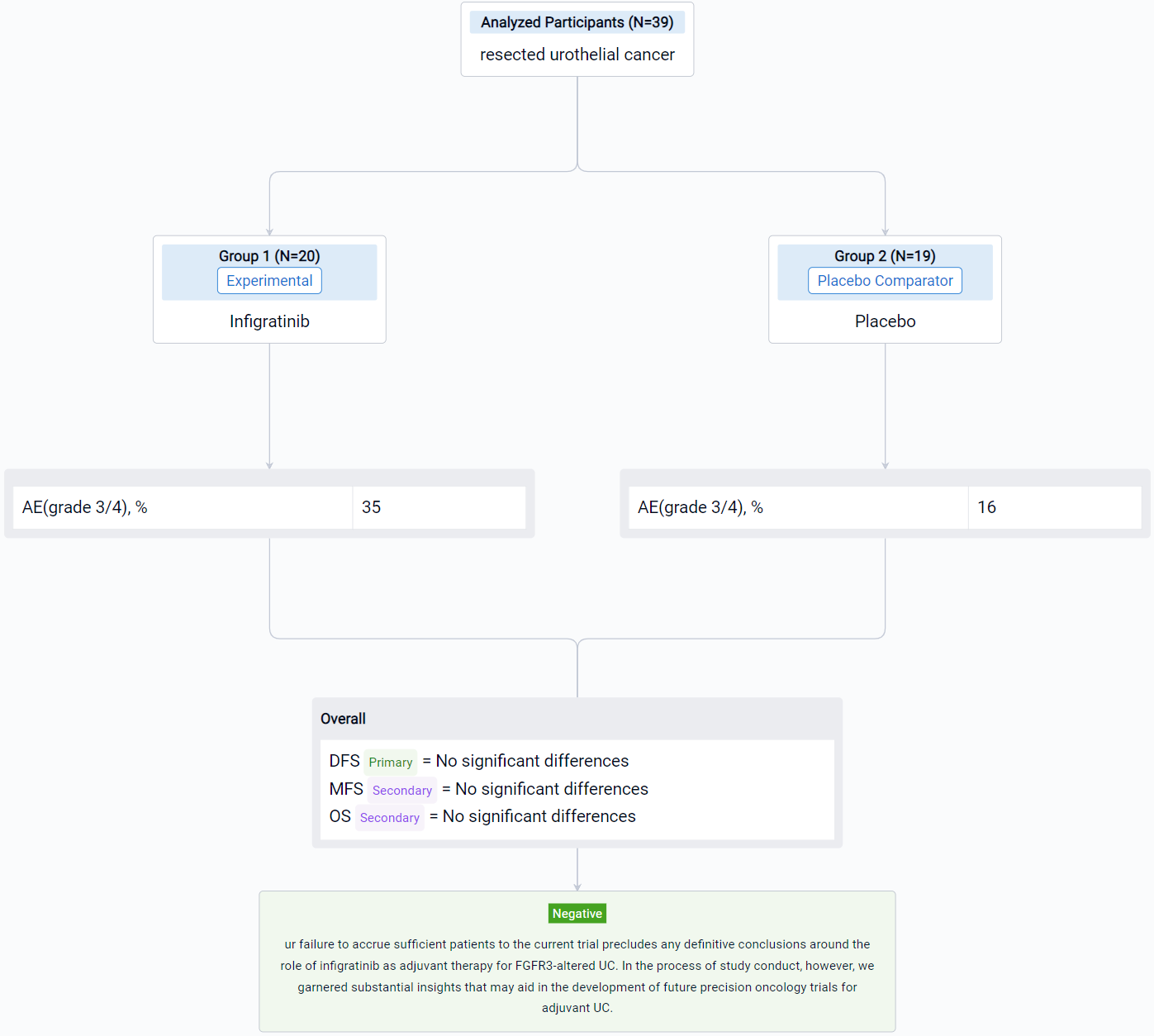
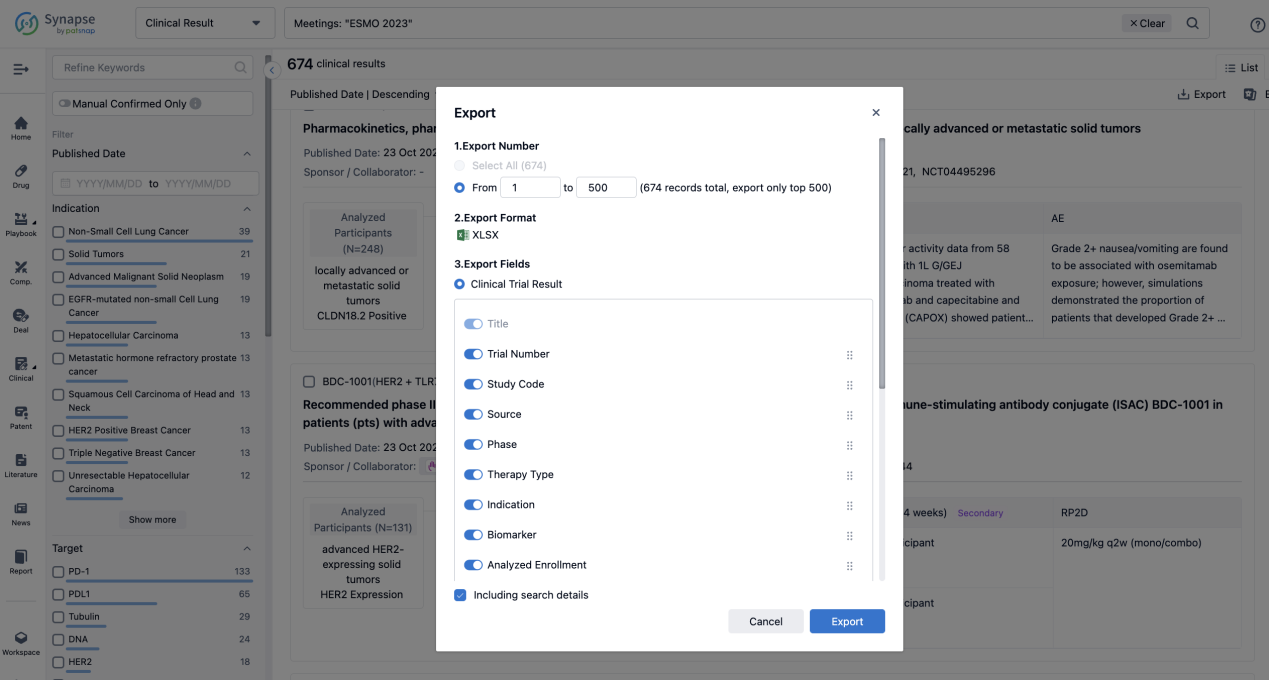
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!