PRGN-2012 - Therapeutic Vaccine for Recurrent Respiratory Papillomatosis
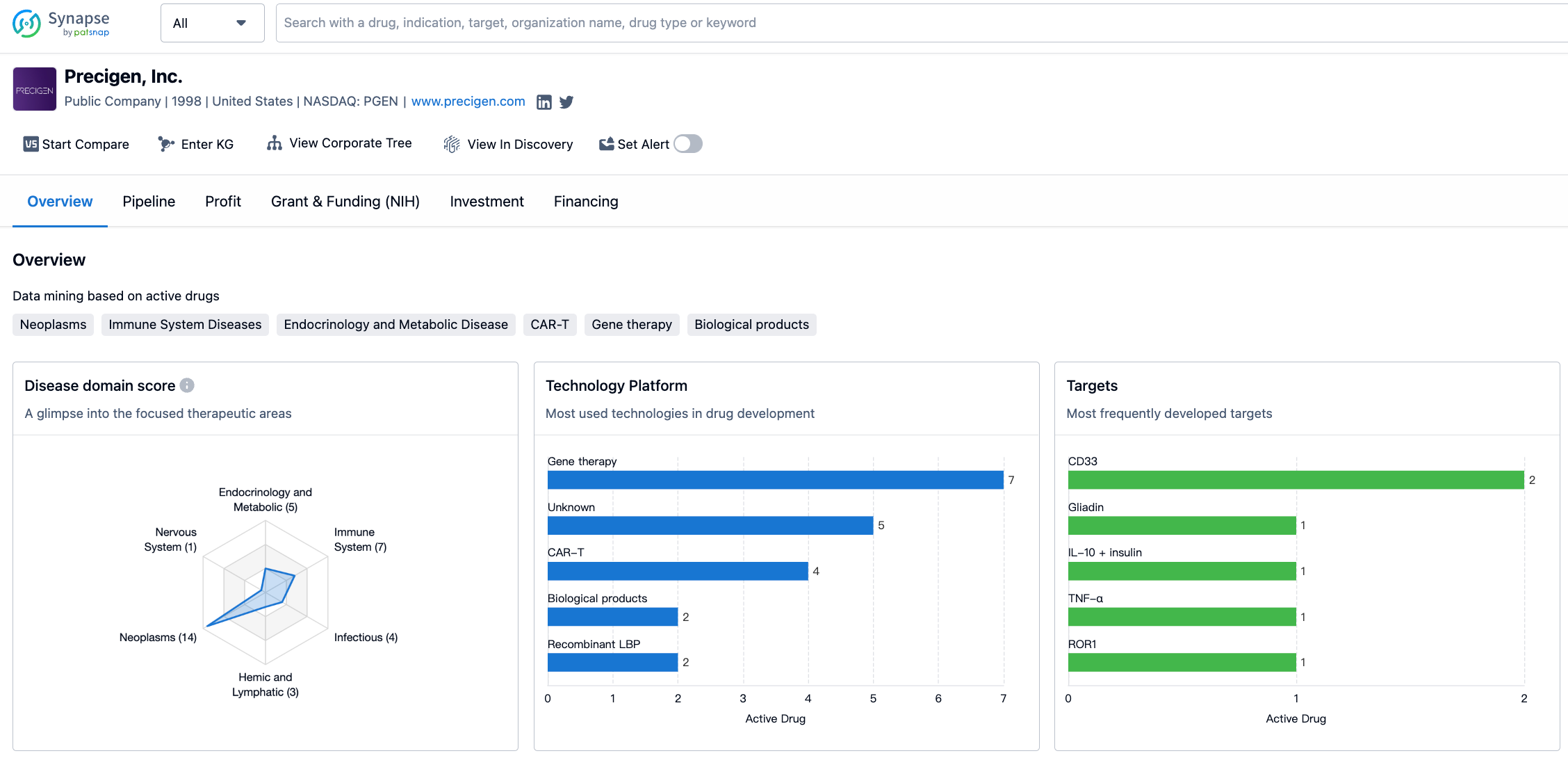
PRGN-2012 is a therapeutic vaccine that achieves therapeutic effects through immune stimulation. Even in clinical phase 2 and still in the receiving state, it has obtained FDA breakthrough therapy certification for the treatment of Recurrent respiratory papillomatosis (RRP). The R&D company is Precigen, Inc., which focuses on gene and cell therapy in core treatment fields such as immune oncology, autoimmune diseases and infectious diseases. The distribution characteristics of its disease fields, technology platforms, targets, etc. are shown in the following figure. For more detailed information on R&D status, core patents, analysis, etc., please click on the image link below.
Mechanism of Action
PRGN-2012 is the first immunotherapy drug for this indication. PRGN-2012 is an innovative therapeutic vaccine with optimized antigen design that uses Precigen’s gorilla adenovector technology, part of Precigen’s proprietary AdenoVerse platform, to elicit immune responses directed against cells infected with human papillomavirus (HPV) type 6 (HPV 6) or HPV type 11 (HPV 11) .
Non-clinical trial
In a vitro study, the stimulation response of PRGN-2012 to T cells was evaluated. T cells were divided into two groups, one from RRP patients and the other from healthy volunteers. The supernate concentrations of IFNγ、granzyme B and GM-CSF are shown below, indicating that PRGN-2012 triggers antigen-specific reactions targeting HPV 6 and HPV 11 in T lymphocytes
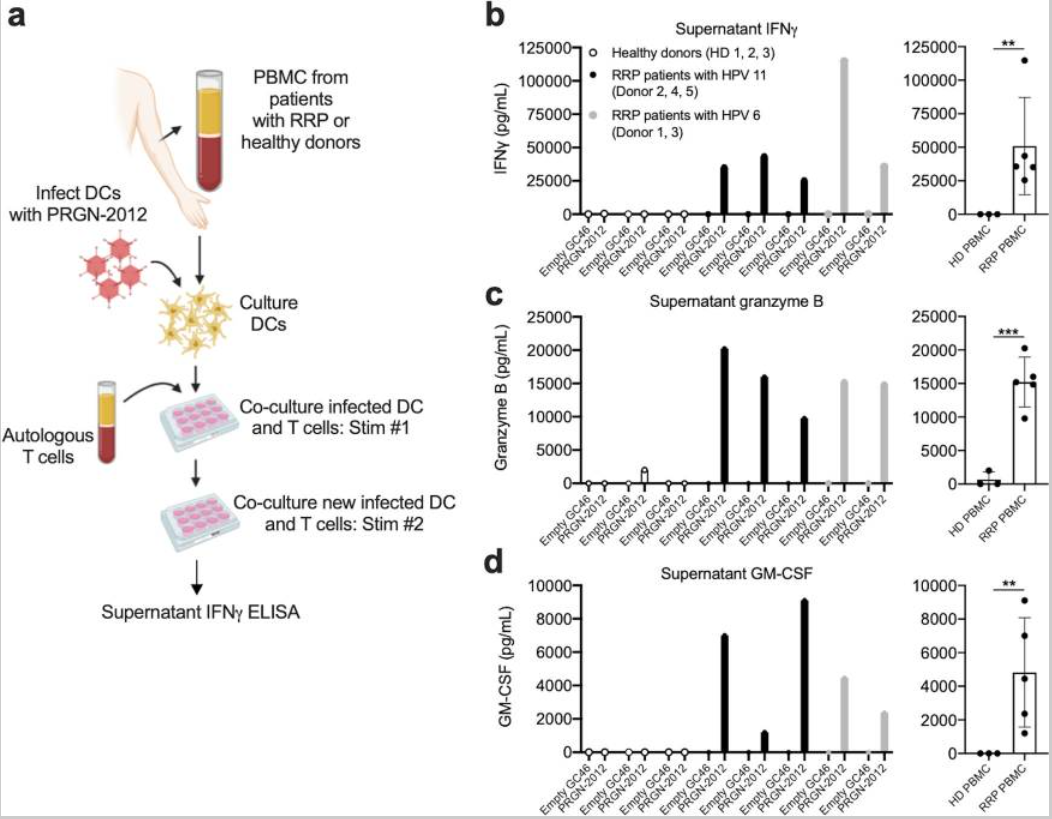
Efficacy and safety
The Breakthrough Therapy Designation was informed by the clinical evidence for PRGN-2012 generated in the Phase 1 study (NCT04724980), which was presented at Precigen's most recent R&D day, and showed strong response at the recommended phase 2 dose (RP2D) in patients who had an average of 5.8 RRP surgeries (range 3 – 10) in the year prior to PRGN-2012 treatment. PRGN-2012 treatment resulted in 50% of patients (6 out of 12) in Complete Response, requiring no post-treatment surgeries with a minimum follow up of 12 months. PRGN-2012 treatment resulted in a reduction of surgeries in 83% (10 out of 12) patients in the 12 months following treatment. PRGN-2012 induced robust de novo HPV-specific T-cell immune response in RRP patients. PRGN-2012 was well-tolerated with no dose-limiting toxicities and no treatment-related adverse events greater than Grade 2.
Competitive Landscape
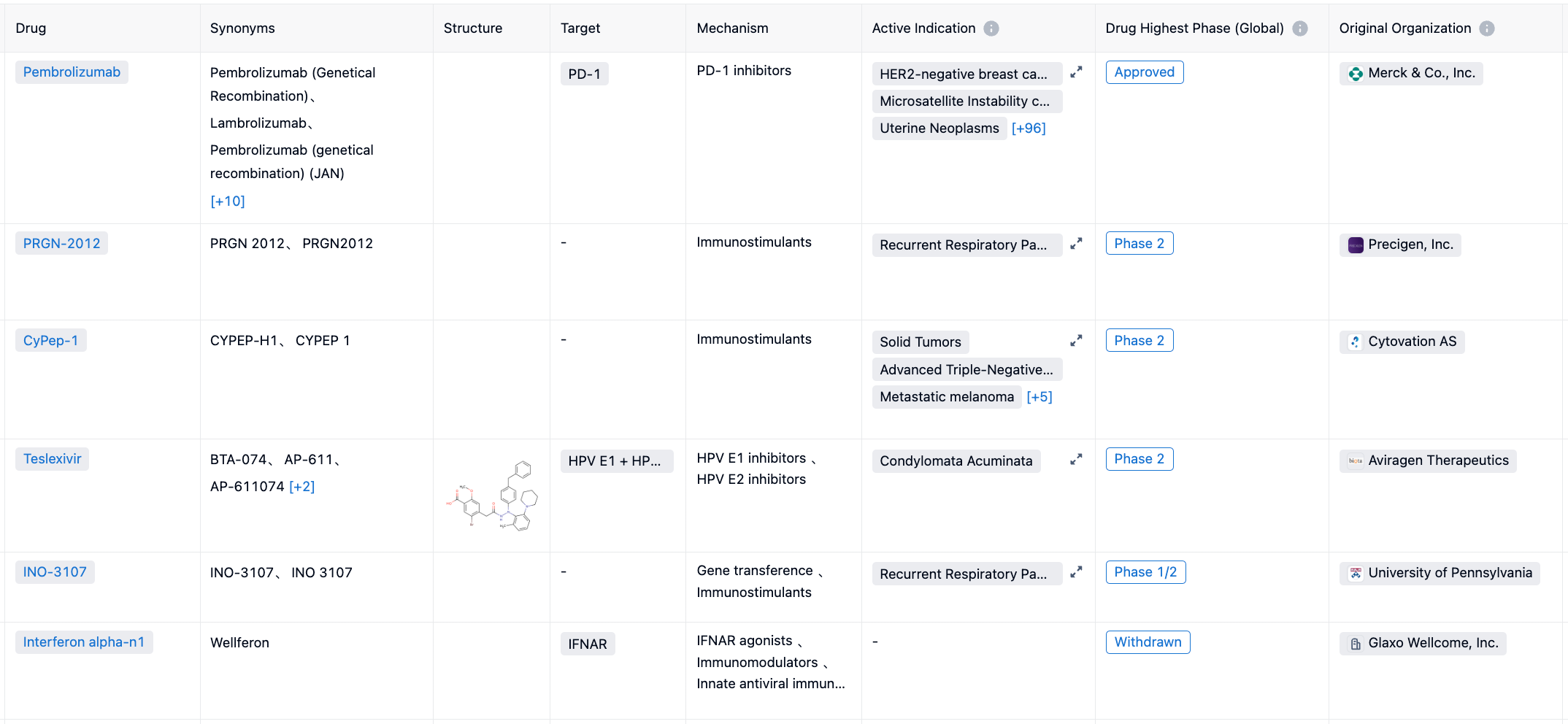
According to “Synapse”, there are RRP drugs, three of which have been terminated. For more information, please click on the image link below.