Promising Phase 1 Results Advance Blue Earth Therapeutics' Lutetium (177Lu) rhPSMA-10.1 Clinical Development
Blue Earth Therapeutics Ltd, a rising authority in the field of therapeutic radiopharmaceuticals, has announced additional promising advancements concerning its innovative investigational radioligand treatments. The enrollment of participants for the Phase 1 trial of Lutetium (177Lu) rhPSMA-10.1 Injection was completed in July. Preliminary findings from this trial indicate a favorable safety profile. Radiation dosimetry assessments conducted over three cycles demonstrated significant tumor-absorbed radiation doses compared to the doses received by crucial normal organs, including the kidneys and salivary glands. Although the final analysis is still in progress, the ratio of radiation dosage delivered to tumors versus that delivered to the kidneys and salivary glands is notably impressive when compared to existing literature on first-generation radioligand therapies.
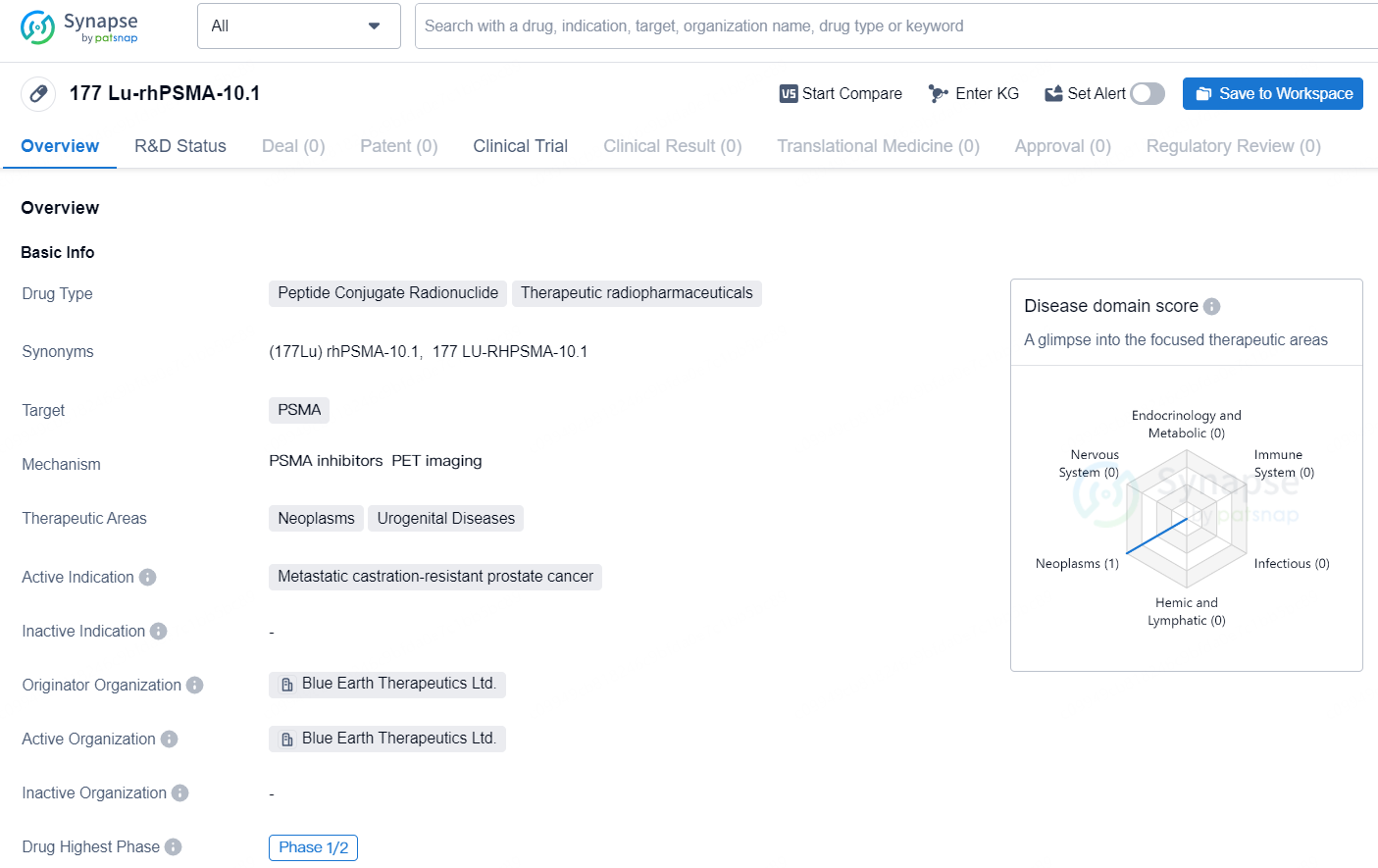
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This information paves the way for the commencement of the Phase 2 segment of the Phase 1/2 trial later this year. The company has provided regulators with the proposed design for the Phase 2 study and, pending approval from the trial safety committee, intends to investigate innovative dosing strategies during this phase. Given the favorable outcomes regarding the absolute uptake in tumors and normal organs observed during Phase 1, the Phase 2 investigation will consider several approaches:
1. Administering a notably higher total injected radioactivity in contrast to the recent Phase 3 studies involving other agents.
2. Implementing a front-loading strategy for the administered radioactivity.
3. Prolonging the duration of radioactivity administration to enhance treatment time.
In conjunction with the positive radiation dosimetry findings from Phase 1 for the Lutetium (177Lu) rhPSMA-10.1 Injection, these elements will further bolster the overarching aim of achieving improved patient outcomes.
"We are thrilled with the recent data, which reinforce our best-in-class perspective and provide a clear trajectory for advancing from Phase 1 to Phase 2 in the development of our leading therapy," stated CEO David Gauden. "We are on course to initiate Phase 2 within the upcoming months, and we anticipate presenting the complete Phase 1 results at a scientific conference in 2025."
"The existing research increasingly suggests that fixed dosing at set intervals may not be the most effective approach. Front-loading radioactivity and extending the duration of therapy could potentially delay disease progression. We aim to investigate these strategies in Phase 2. Our goal is to refine dosing now in order to secure optimal outcomes for patients in future pivotal trials," remarked Dr. Daniel Stevens, Head of Clinical Development and Medical at Blue Earth Therapeutics. "We believe that tailoring dosing based on individual patient data will be crucial for improved results."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 11, 2024, there are 292 investigational drugs for the PSMA targets, including 271 indications, 295 R&D institutions involved, with related clinical trials reaching 4269, and as many as 28237 patents.
177 Lu-rhPSMA-10.1 is a peptide conjugate radionuclide categorized as a therapeutic radiopharmaceutical. The drug targets prostate-specific membrane antigen (PSMA) and is indicated for the treatment of metastatic castration-resistant prostate cancer. Its therapeutic areas of focus include neoplasms and urogenital diseases. Originating from Blue Earth Therapeutics Ltd., the drug has reached the highest global phase of Phase 1/2 clinical trials.