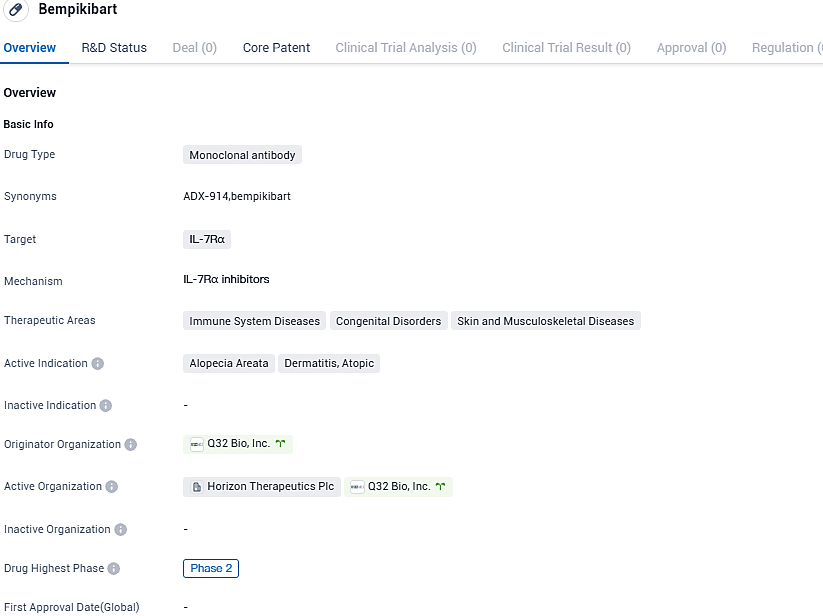
Q32 Bio retrieves global rights for Bempikibart (ADX-914) back from Amgen
Biotech firm Q32 Bio, which is currently in the clinical phase and is focused on creating biologic therapies to balance immune responses, has revealed plans to take back complete developmental and commercial rights for bempikibart (previously known as ADX-914) from Amgen.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the year 2022, a partnership contract with an option to produce bempikibart for autoimmune disorders was signed by Q32 Bio and Horizon Therapeutics. According to the agreement, Q32 Bio received a preliminary payment of $55 million and progressive financial support to complete two ongoing Phase 2 studies, after which Horizon obtained the right to purchase the project at a predetermined cost.
Bempikibart is a completely human anti-IL-7Rα protein, which restores the function of the adaptive immune system by blocking signals transmitted by both IL-7 and TSLP. Currently, two phase 2 studies are being conducted to examine its effect on atopic dermatitis and alopecia areata. The data of the phase 2 trials is yet to be unblinded, and the estimated time for the release of the initial phase 2 results would be the latter half of 2024 as revealed by Q32 Bio.
"The fact that we have regained rights to bempikibart in the current phase 2 trials enthuses us greatly," expressed Jodie Morrison, CEO of Q32 Bio. "Bempikibart's blocking of IL-7 and TSLP could possibly provide a unique and transformative alternative for treating autoimmune and other inflammatory ailments with high unmet needs. Our team is dedicated to swiftly advancing the Phase 2 clinical trials for both atopic dermatitis (AD) and alopecia areata (AA) and are looking forward to the unblinding and release of the initial results in the latter part of the following year."
Pursuant to the terms of the contract for getting back the rights from Amgen, Q32 Bio retains the initial payment and any funding received for development. Q32 Bio will remunerate Amgen upon obtaining the first regulatory consent and following achievement of commercial milestones based on yearly net sales quotas.
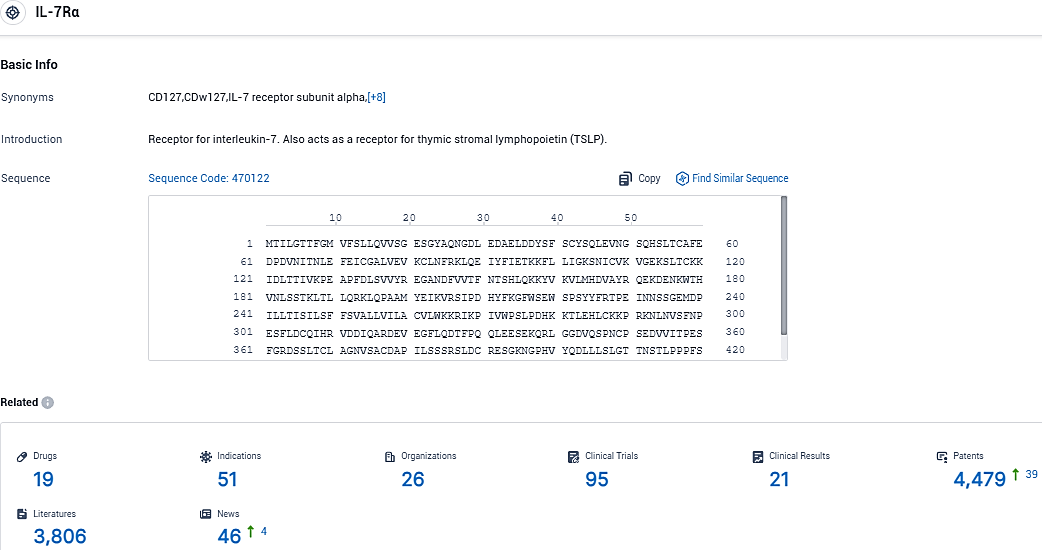
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 23, 2023, there are 19 investigational drugs for the IL-7Rα target, including 51 indications, 26 R&D institutions involved, with related clinical trials reaching 95, and as many as 4479 patents.
Bempikibart shows potential for treating immune system diseases, congenital disorders, and skin and musculoskeletal diseases, specifically alopecia areata and dermatitis, atopic. Further research and clinical trials will be necessary to determine its safety and efficacy in treating these conditions.